Composition for treating carbapenem antibiotic resistant acinetobacter baumannii infection
A technology of Acinetobacter baumannii and carbapenems, which is applied in the field of medicine and can solve problems such as lack of antibiotic compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
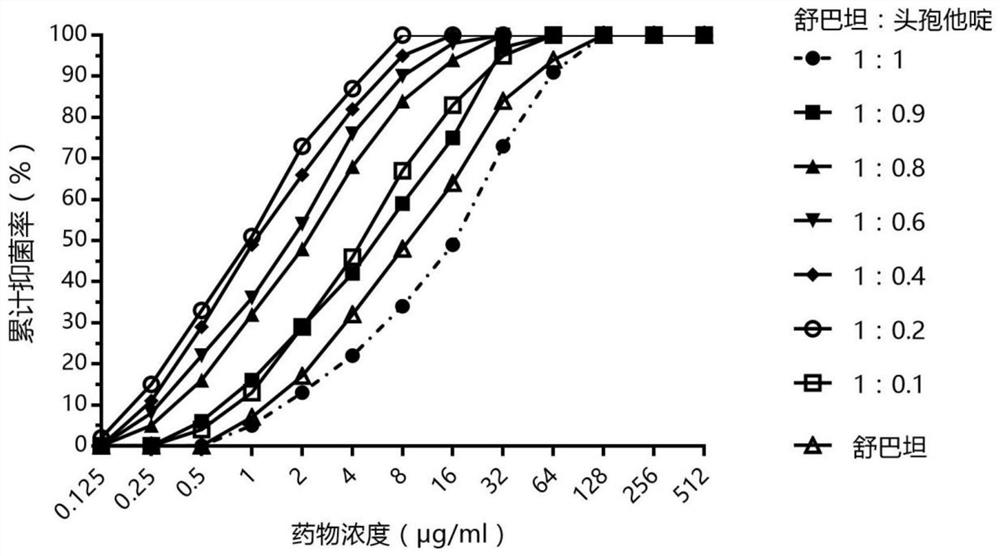
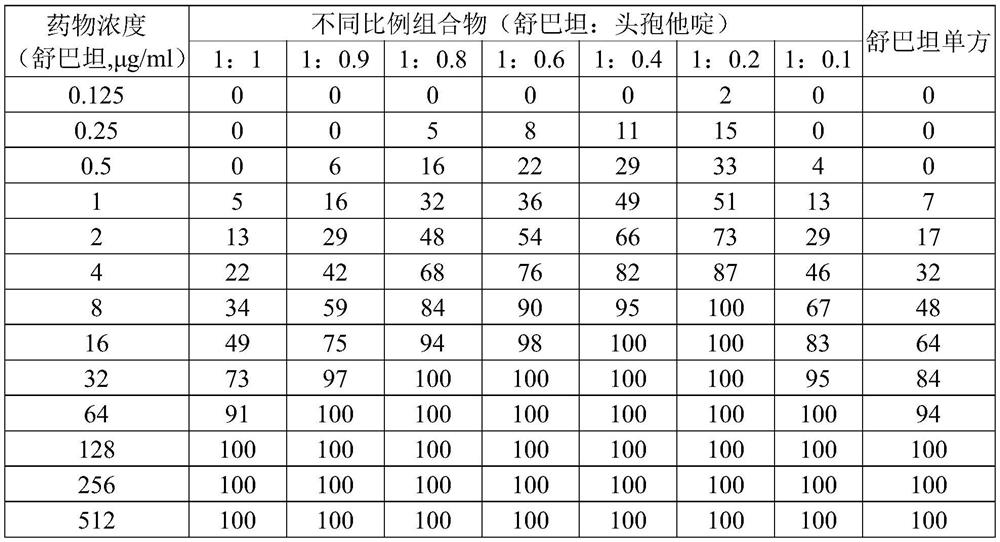
[0053] Example 1: In vitro bacteriostatic effect of sulbactam and low proportion of ceftazidime on drug-resistant Acinetobacter baumannii
[0054] Strains: 157 clinical isolates of Acinetobacter baumannii resistant to carbapenem antibiotics were selected and named CR-Ab. The selection criteria are: clinically isolated Acinetobacter baumannii, the drug susceptibility test is carried out with imipenem and meropenem, which are commonly used carbapenems, according to the CLSI paper method. One or two drug resistances in Nanzhong were selected as inclusion criteria. Resistance refers to the MIC of imipenem or / and meropenem ≥ 8 μg / ml according to the criteria of Table 2B-2 of CLSI-M100 27th.
[0055] Drugs: take sulbactam and ceftazidime, and prepare the compositions in different proportions. The method is to fix the amount of sulbactam and gradually reduce the amount of ceftazidime to obtain the weight ratios of sulbactam and ceftazidime of 1:1, 1:0.9, 1:0.8, 1:0.6, 1:0.4, 1:0.2 ...
Embodiment 2
[0061] Example 2: In vitro long-term bactericidal effect of sulbactam in combination with low-proportion ceftazidime on drug-resistant Acinetobacter baumannii
[0062] Strain: carbapenem-resistant Acinetobacter baumannii CR-Ab in Example 1.
[0063] Medicines: four sulbactam-ceftazidime compositions with ratios of 1:1, 1:0.8, 1:0.4 and 1:0.2 prepared in Example 1, respectively.
[0064] Methods: Five CR-Ab strains were selected, and each composition was used for drug susceptibility tests to obtain their respective MIC values. Then the strain was cultured with MH broth to obtain a concentration of 10 6 CFU / mL of bacteria was used as the inoculum. Take the bacterial inoculation mother liquor respectively, add each medicine respectively, make the final concentration of the medicine (calculated by the concentration of sulbactam) respectively 0 (negative control), 0.25MIC, 1MIC, 2MIC, 4MIC, 8MIC, 16MIC, respectively, at 0, Samples were taken at each time point of 2, 4, 6, 8, 12,...
Embodiment 3
[0076] Example 3: Protective effect of sulbactam in combination with low proportion of ceftazidime on pneumonia model animals infected with drug-resistant Acinetobacter baumannii
[0077] Drug: each composition of sulbactam and ceftazidime used in Example 1 in the ratio of 1:1, 1:0.9, 1:0.8, 1:0.6, 1:0.4, 1:0.2 and 1:0.1, with water for injection It is formulated into a drug solution; the commercially available meropenem is formulated into a drug solution with water for injection; the commercially available meropenem, plus sulbactam, is formulated into a 1:1 drug solution of meropenem and sulbactam with water for injection.
[0078] Strain: Use CR-Ab strain, cultivated to a concentration of 10 8 CFU / mL bacterial solution.
[0079] Animals: Male C57BL / 6 mice, randomly divided into normal control group, model control group, meropenem group, meropenem-sulbactam 1:1 group, sulbactam-ceftazidime 1:1 group, and sulbactam-ceftazidime 1:0.9 group , Sulbactam-ceftazidime 1:0.8 group,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com