Hyperbranched phosphorescent polymer and preparation method thereof
A technology of polymers and compounds, applied in the field of hyperbranched phosphorescent polymers and their preparation, can solve the problems of widening optical band gap, unsatisfactory electroluminescence efficiency, concentration quenching effect, etc. The effect of improving electroluminescence efficiency and improving electroluminescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 4-iodophenol (220mg, 1.00mmol), K 2 CO 3 (276mg, 2.00mmol) and a solution of (4-((10-bromodecyl)oxy)cyclohexyl)benzene (396mg, 1.00mmol) dissolved in 20mL of DMF were added to a 50ml three-necked flask and heated at 110°C Heat for 16 hours. After cooling to room temperature, dichloromethane was added to the reaction mixture. with H 2 The organic layer was washed with O and washed with Na 2 SO 4 dried, and the dried residue was removed from CH 2 Cl 2 / methanol (volume ratio is 4:1) and precipitates out, obtains white solid I-Y, (451mg, productive rate 84.0%), and reaction equation is:
[0029]
Embodiment 2
[0031] N-(3-aminophenyl)-2,7-dibromocarbazole (532 mg, 1 mmol), I-Y (1541.6 mg, 2.8 mmol), Pd(OAc) 2 (12 mg, 0.06 mmol), t-BuONa (384 mg, 4.0 mmol), toluene (10 mL) and 10 wt% P(t-Bu) dissolved in hexane 3 (290mL, 1mmol) was added into a 500ml three-necked flask, stirred at a speed of 300r / min and reacted at 120°C for 48h. After cooling to room temperature, petroleum ether was added to the reaction mixture. The organic layer was washed with toluene and washed with Na 2 SO 4 dry. After evaporation, the residue was dissolved in petroleum ether / CH 2 Cl 2 (7:1) is carried out silica gel column chromatography purification as eluent, obtains the compound shown in formula (II) (500mg, productive rate 70.0%), and chemical reaction equation is:
[0032]
Embodiment 3
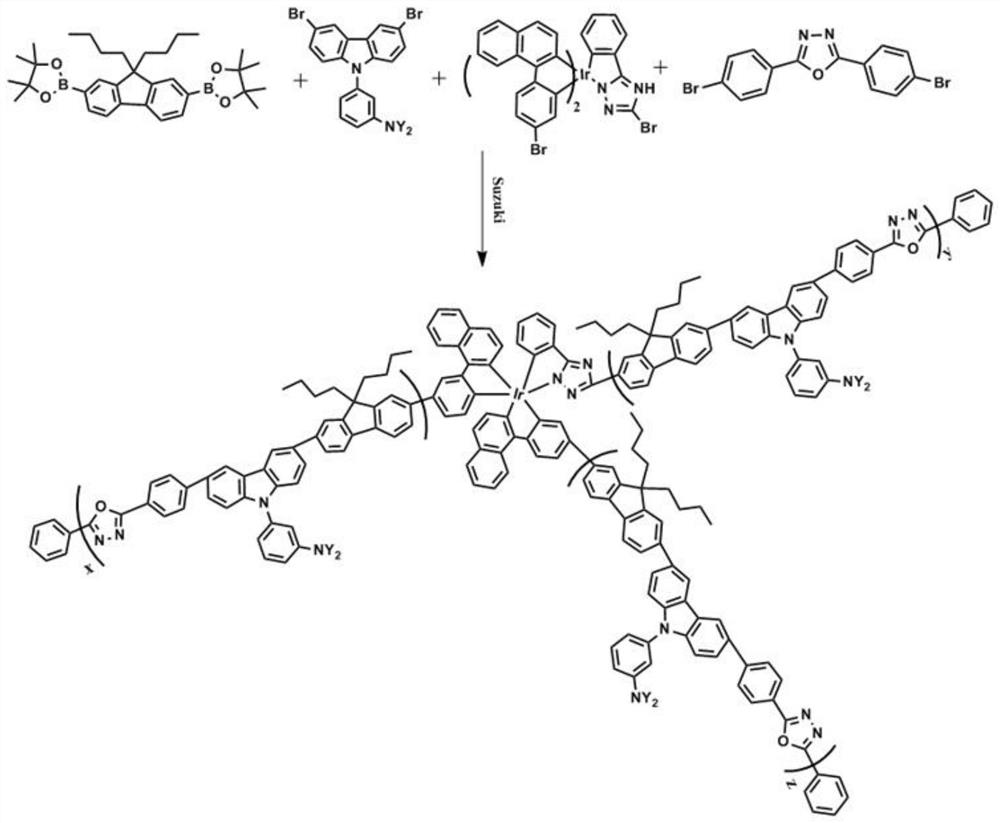
[0034] 2,2'-(9,9-dibutyl-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborin alkane) (265.18mg, 0.5mmol), compound represented by formula (II) (491.736mg, 0.4mmol), 2,5-bis(4-bromophenyl)-1,3,4-oxadiazole (19.0mg , 0.05mmol), the compound represented by formula (III) (19.6mg, 0.02mmol), 10mL of purified toluene and 10mL of dehydrated tetrahydrofuran were added to a 50mL two-necked flask, and 5mg of Pd(OAc) was sequentially added under the protection of argon. 2 and 6mgPCy 3 , heated to 80°C and reacted for 2 hours, added 6mL of 20% organic base, refluxed for 36 hours, then added 2mgPCy 3 and 1mgPd(OAc) 2 Capping, after reacting for 10h, inject 0.3mL bromobenzene to capping again. After 12 hours, the reaction liquid was precipitated in methanol, and then extracted with methanol and acetone for 12 hours respectively, and the obtained concentrated extract was precipitated in methanol. The precipitate was dissolved in toluene, and carried out silica gel column chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com