Acceptor material based on benzoimide, and preparation method and application of acceptor material
A technology of benzimide and acceptor materials, which is applied in semiconductor/solid-state device manufacturing, electric solid-state devices, semiconductor devices, etc., can solve the problems of narrow absorption band, wide optical bandgap, weak absorption, etc., and increase space Twisting, reducing the optical bandgap, reducing the effect of aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The embodiment of the present invention also provides a preparation method of a benzimide-based acceptor molecule, comprising the following steps:
[0063] S10. Obtaining an aldehyde compound and an electron-withdrawing compound based on benzimide;
[0064] S20. Obtain a weakly basic catalyst, dissolve the catalyst, the aldehyde compound based on benzimide, and the electron-withdrawing compound in an organic solvent, and reflux for 12 under the condition of 60° C. to 70° C. ~24 hours, get the acceptor molecule based on benzimide;
[0065]Wherein, the aldehyde compound based on benzimide is selected from:
[0066] Compound BH or compound NH
[0067] The electron-withdrawing compound is selected from any one of the following compounds:
[0068]
[0069] Among them, R 1 , R 2 , R 3 are independently selected from C 6 ~C 30 The alkyl chain of, Y is selected from sulfur or selenium, R 4 selected from any one of hydrogen, fluorine, chlorine, cyano, methoxy, su...
Embodiment 1
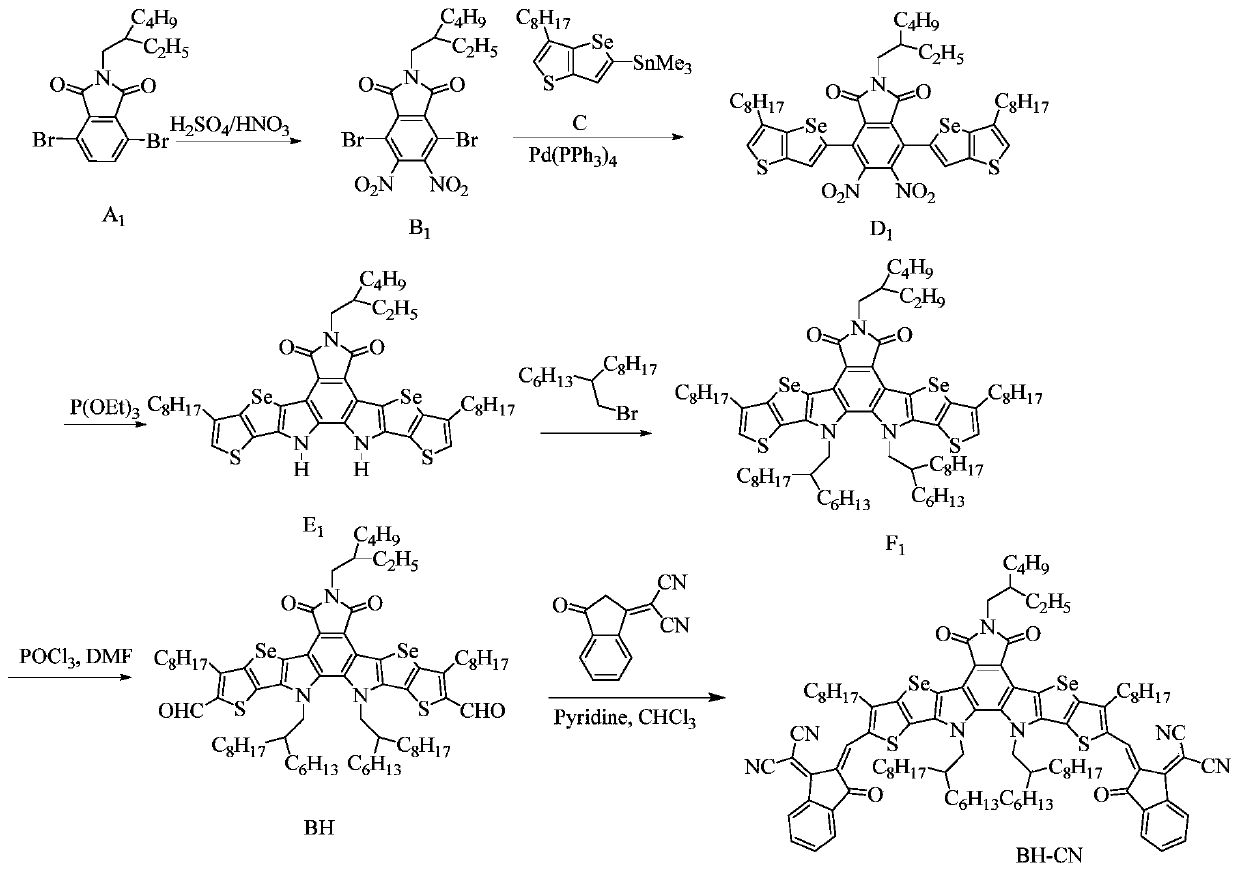
[0129] A kind of preparation method of acceptor molecule BH-CN based on benzimide, as attached figure 1 The synthetic route is shown as:
[0130] S10. Compound A 1 Synthesis of aldehyde compound BH based on benzimide as starting material.
[0131] ①Compound A 1 (4.3g, 10.3mmol) was dissolved in concentrated sulfuric acid / fuming nitric acid (30mL / 75mL), refluxed at 90°C for 15 hours, then cooled to room temperature, and then gradually poured into ice water. Sodium hydroxide was added to the solution. Afterwards, the precipitate was filtered out, and then purified with a silica gel column (mobile phase: petroleum ether and dichloromethane) to obtain a white solid B 1 (3.4 g, 65.1%), MS (EI, m / z) 506.9.
[0132] ②Compound B 1 (3.4 g, 6.7 mmol) and trimethyl-(6-octylthienoselenophene)tin (11.1 g, 24.0 mmol) were dissolved in 100 mL of anhydrous toluene. Ar 2 Under protection, the Pd(PPh 3 ) 4 (0.8g, 0.69mmol) was added to the reaction solution, and stirred at reflux at...
Embodiment 2
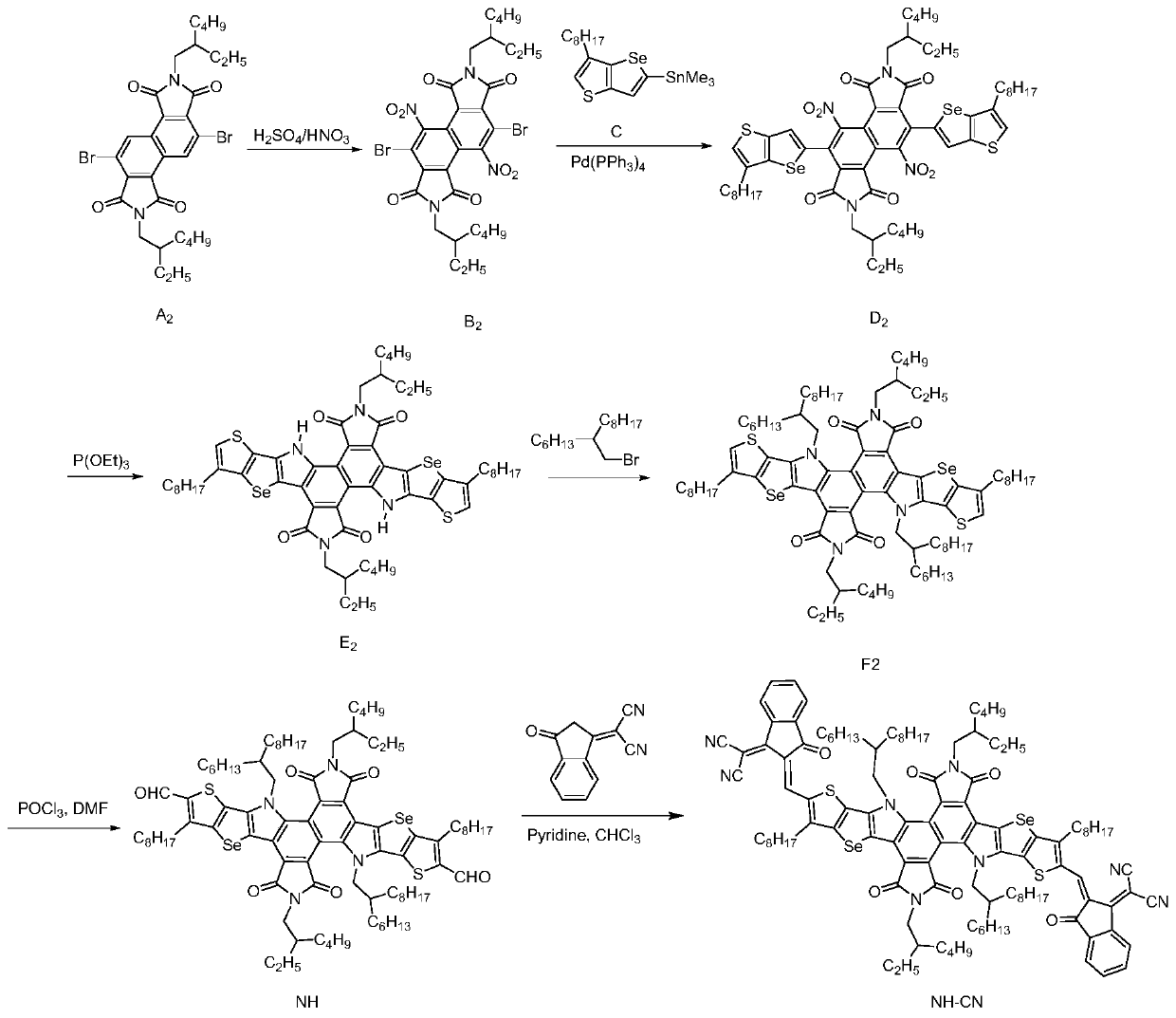
[0140] A kind of preparation method of acceptor molecule NH-CN based on benzimide, as attached figure 2 The synthetic route is shown as:
[0141] S10. Compound A 2 Synthesis of aldehyde compound NH based on benzimide as starting material.
[0142] ①Compound A 2 (4.1g, 6.3mmol) was dissolved in concentrated sulfuric acid / fuming nitric acid (30mL / 75mL), refluxed at 90°C for 15 hours, then cooled to room temperature, and then gradually poured into ice water. Sodium hydroxide was added to the solution. Afterwards, the precipitate was filtered out, and then purified with a silica gel column (mobile phase: petroleum ether and dichloromethane) to obtain compound B as a white solid 2 (3.4 g, 4.6 mmol, 73.0%), MS (EI, m / z) 736.1.
[0143] ②Compound B 2 (3.4 g, 4.6 mmol) and trimethyl-(6-octylthienothiophene)tin (10.5 g, 22.7 mmol) were dissolved in 100 mL of anhydrous toluene. Ar 2 Under protection, the Pd(PPh 3 ) 4 (0.8g, 0.69mmol) was added to the reaction solution, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com