Photosensitizer probe as well as preparation method and application thereof
A photosensitizer and probe technology, applied in the field of biochemistry, can solve the problems of low singlet oxygen quantum yield and poor photostability, and achieve the effects of high-efficiency singlet oxygen production capacity, simple and safe preparation method, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
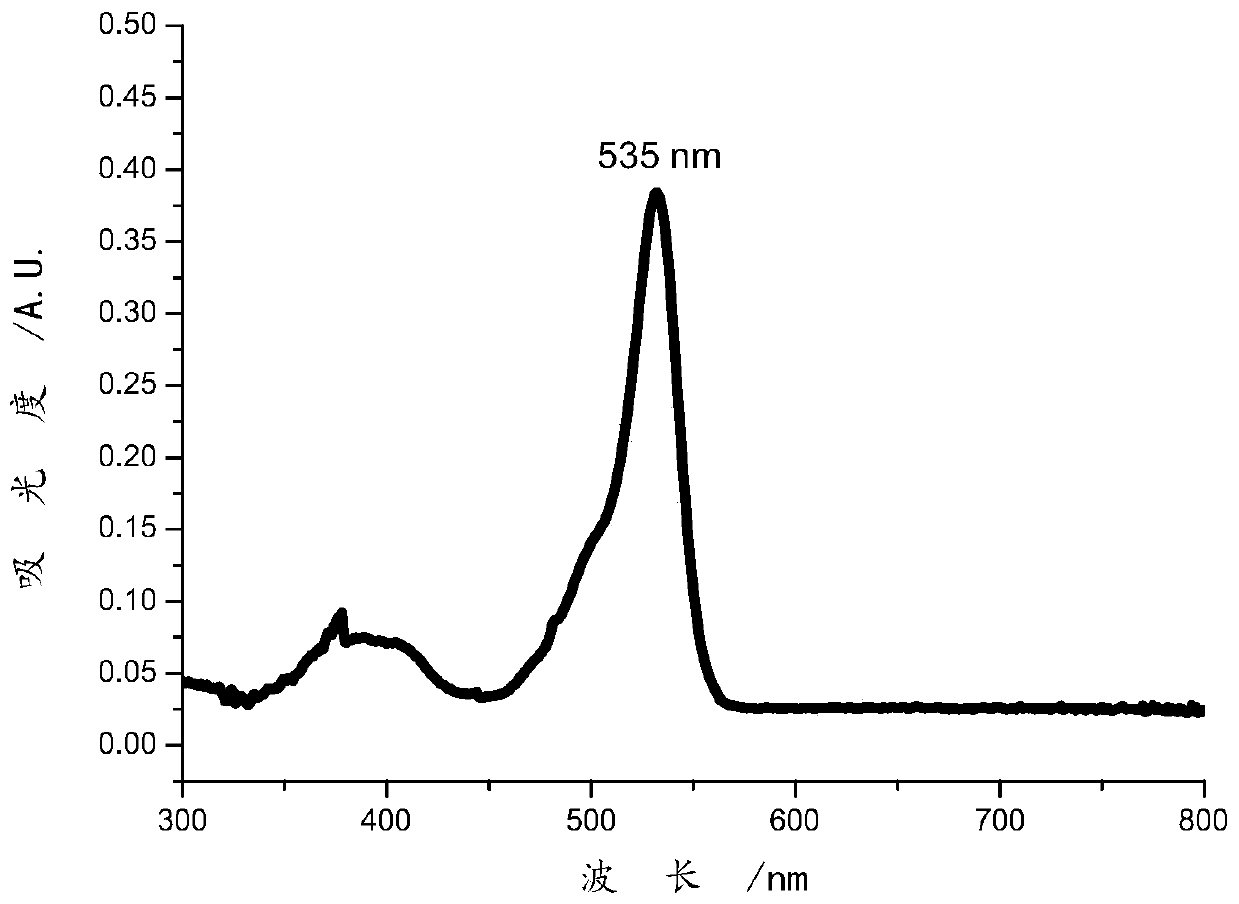
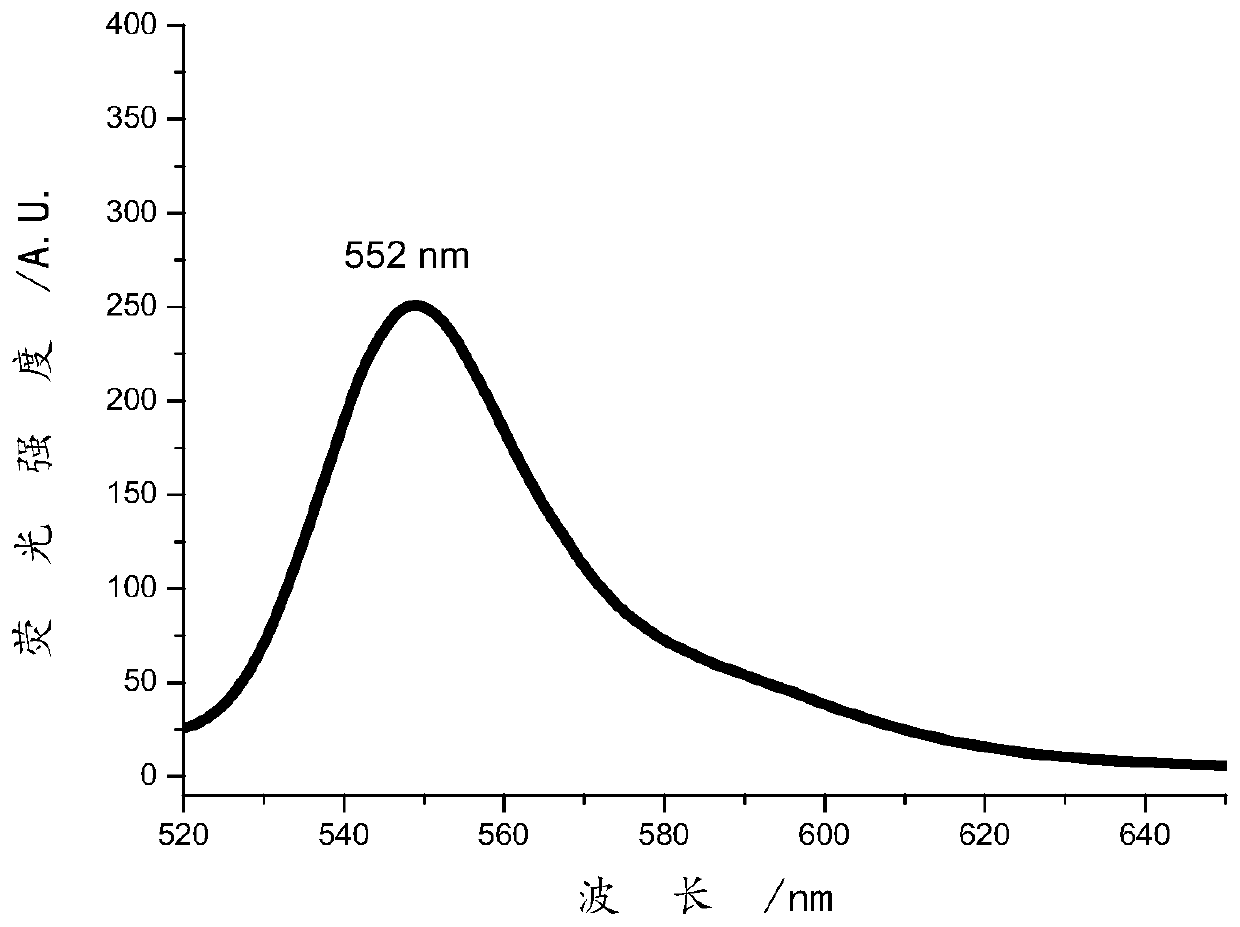
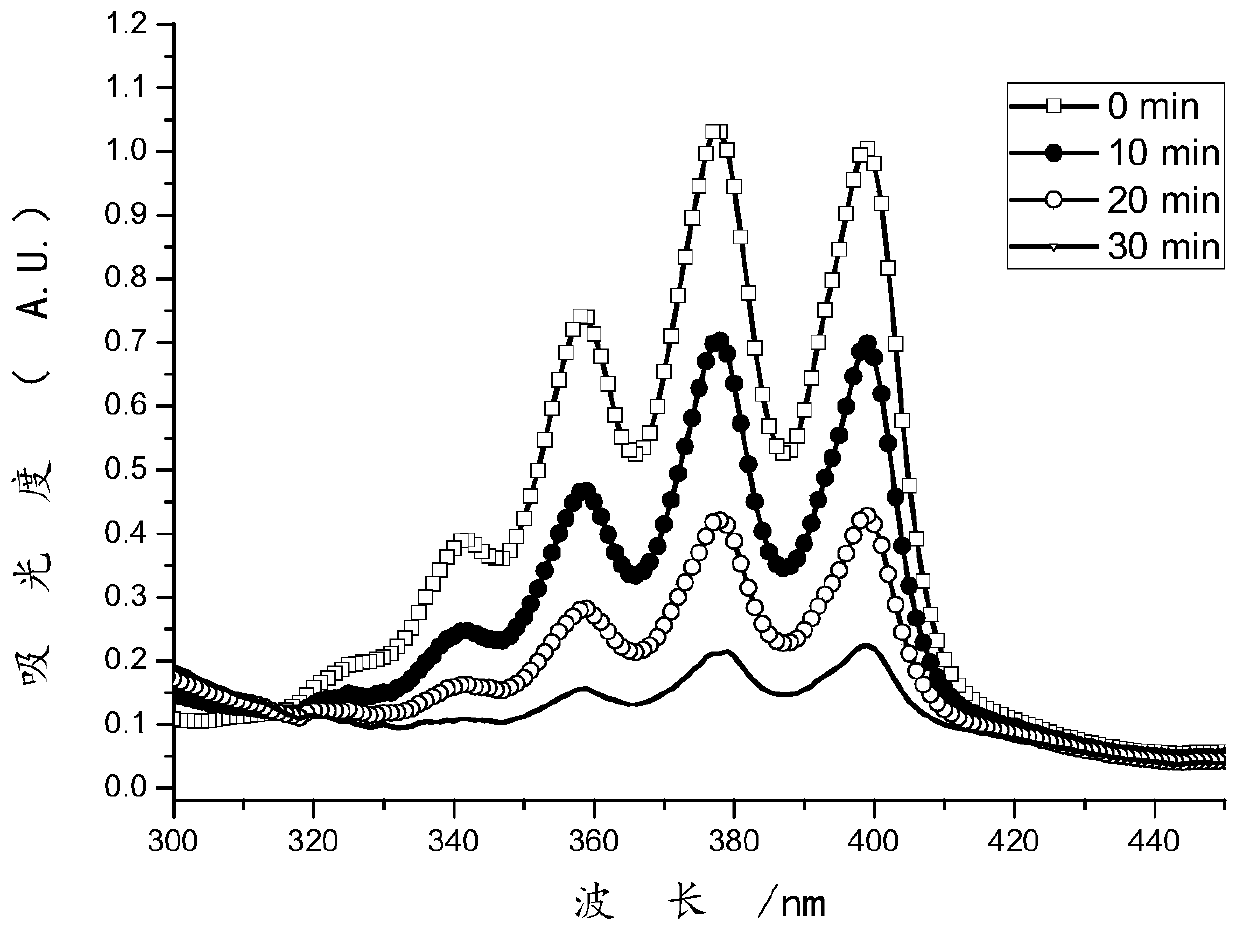
Image
Examples
Embodiment 1
[0032] (1) Under nitrogen atmosphere, dissolve p-nitrobenzaldehyde (1.5g, 10mmol), 2,4-dimethylpyrrole (2.1g, 22mmol) and trifluoroacetic acid (0.2mL) in tetrahydrofuran (30mL) , stirring overnight to obtain the first reaction solution;
[0033] Add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (2.3g, 10mmol, DDQ) into tetrahydrofuran (20mL) to obtain a tetrahydrofuran solution, and add the tetrahydrofuran solution to the first reaction solution Dissolved and reacted for 2 to 4 hours, quickly added triethylamine (20mL) and boron trifluoride diethyl ether complex (BF 3 : 46.5%, 30mL), then transferred to room temperature and reacted for 4 to 12 hours to obtain the reaction solution;
[0034] The solvent of the reaction solution was removed: the solvent was concentrated, the residue was layered with dichloromethane (100mL) and saturated sodium bicarbonate (100mL), the aqueous phase was extracted with dichloromethane (20mL×3), and the combined organic phases were separated in After...
Embodiment 2
[0044] (1) Under nitrogen atmosphere, dissolve p-nitrobenzaldehyde (1.5g, 10mmol), 2,4-dimethylpyrrole (21mmol) and trifluoroacetic acid (0.1mL) in tetrahydrofuran (28mL), and stir for 2h Obtain the first reaction solution;
[0045] Add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, 9mmol) into tetrahydrofuran (10mL) to obtain a tetrahydrofuran solution, add the tetrahydrofuran solution to the first reaction solution to dissolve and After reacting for 2 hours, triethylamine (10 mL) and boron trifluoride etherate (BF 3 : 40%, 20mL), then transferred to room temperature and reacted overnight to obtain a reaction solution;
[0046] The solvent of the reaction solution was removed: the solvent was concentrated, the residue was layered with dichloromethane (100mL) and saturated sodium bicarbonate (100mL), the aqueous phase was extracted with dichloromethane (20mL×3), and the combined organic phases were separated in After the drying of sodium sulfate in water, concentrate and re...
Embodiment 3
[0056] (1) Under nitrogen atmosphere, p-nitrobenzaldehyde (1.5g, 10mmol), 2,4-dimethylpyrrole (23mmol) and trifluoroacetic acid (0.3mL) were dissolved in tetrahydrofuran (32mL), stirred for 24 Obtain the first reaction solution;
[0057] Add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (11mmol, DDQ) into tetrahydrofuran (22mL) to obtain a tetrahydrofuran solution, add the tetrahydrofuran solution to the first reaction solution to dissolve and After reacting for 6 hours, triethylamine (30mL) and boron trifluoride etherate (BF 3 : 50%, 40mL), then transferred to room temperature and reacted for 24h to obtain the reaction solution;
[0058] The solvent of the reaction solution was removed: the solvent was concentrated, the residue was layered with dichloromethane (100mL) and saturated sodium bicarbonate (100mL), the aqueous phase was extracted with dichloromethane (20mL×3), and the combined organic phases were separated in After the drying of sodium sulfate in water, concentrate a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com