Endonuclease sexing and sterilization in insects
An endonuclease and insect technology, applied in the direction of nucleic acid vectors, hydrolytic enzymes, biochemical equipment and methods, etc., can solve the problem of limiting the wide applicability of other species, unable to release infected females, and damaging the health of RIDL/fsRIDL males And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
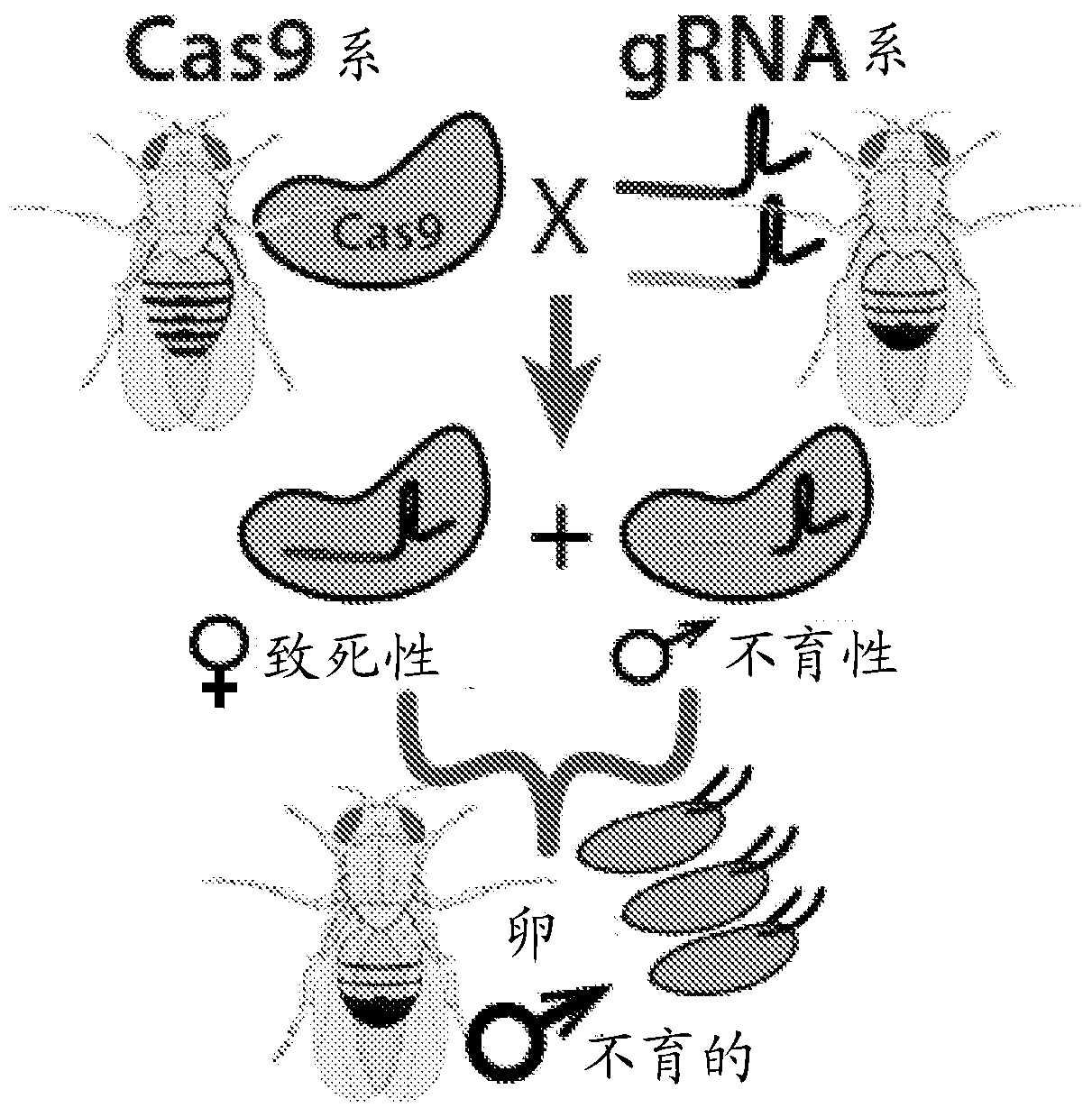
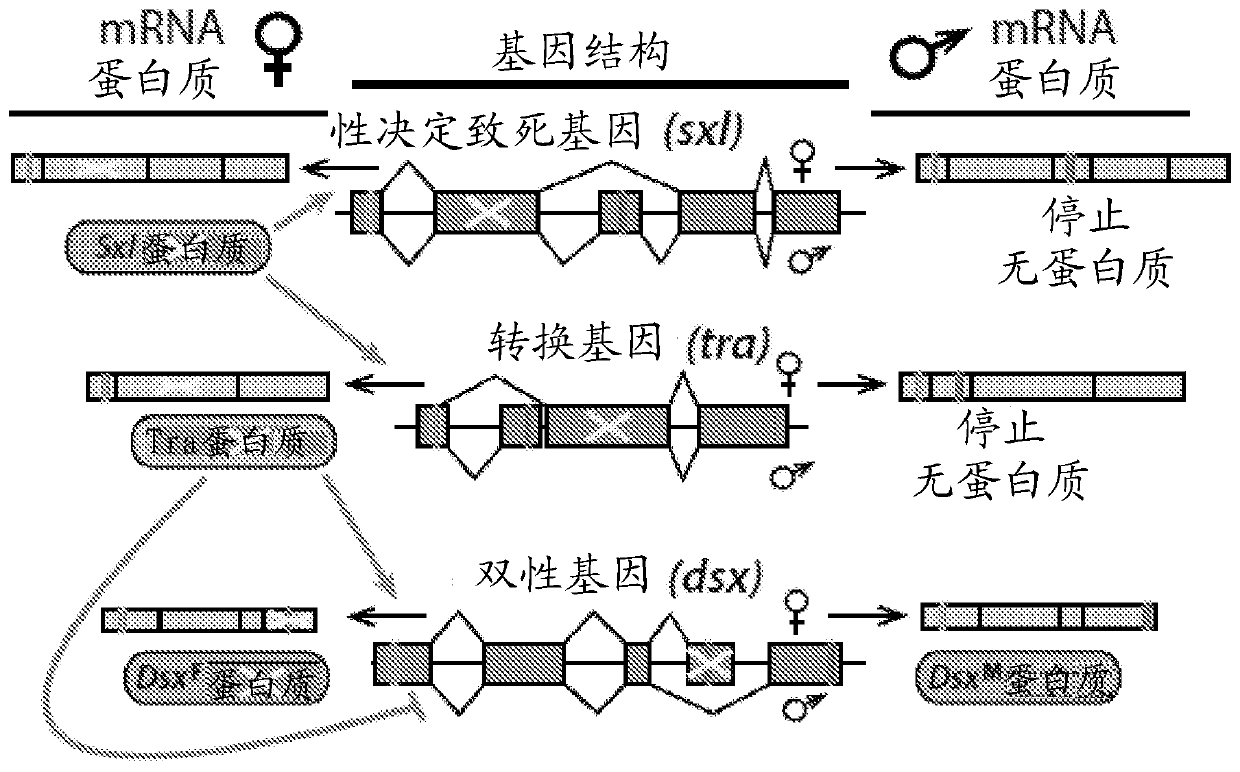
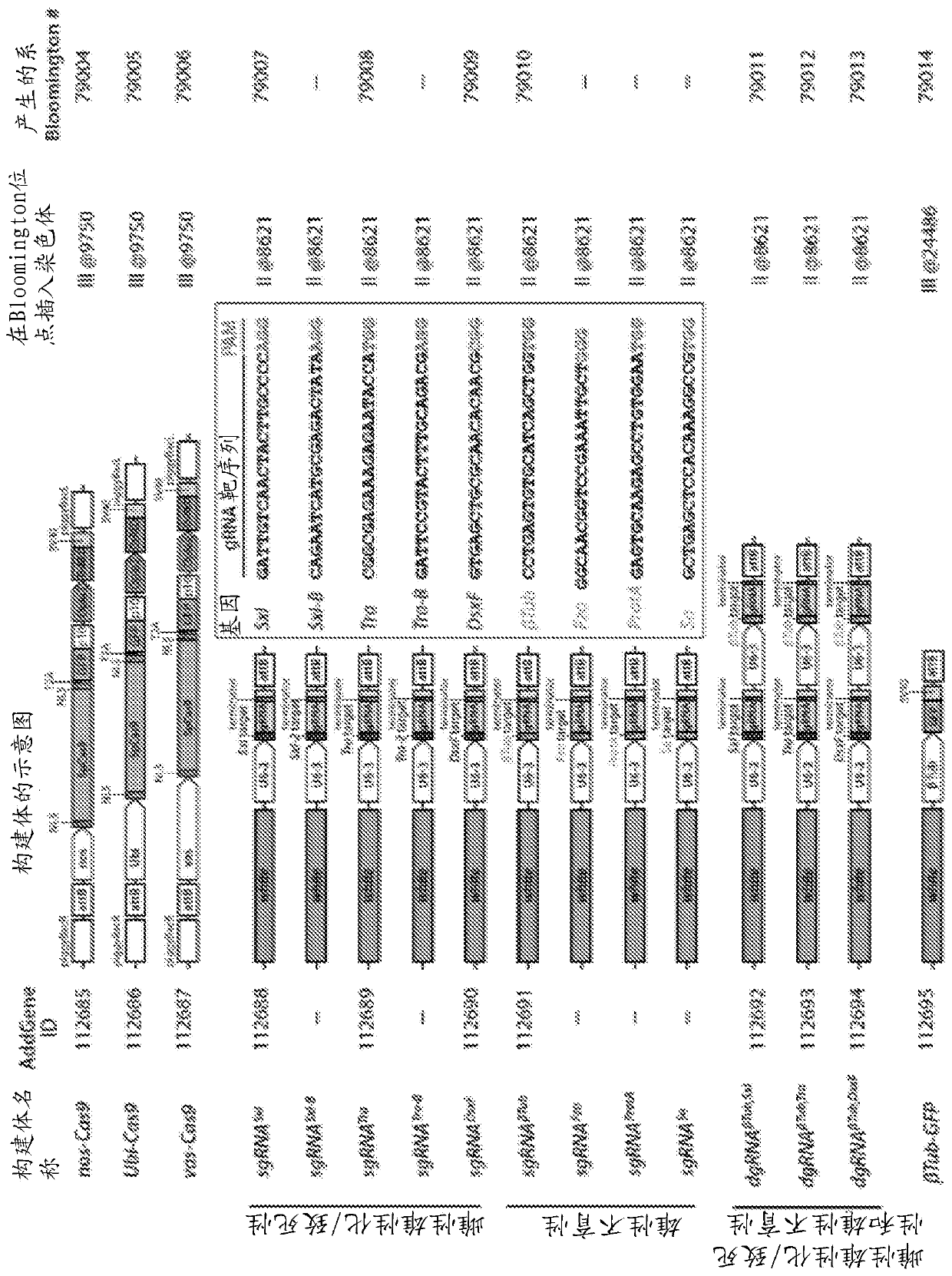
[0099] Example 1. Binary CRISPR-induced female masculinization / lethality or male sterility. To engineer pgSIT, single guide RNA (sgRNA) and spCas9 (from here Cas9) expression lines were generated in Drosophila. A total of 9 homozygous sgRNA lines were developed to target genes essential for female viability or genes important for male fertility. For female viability, these genes include sex-specific alternatively spliced sex-determining genes, which include eg Figures 1B-1C As shown in and in Slee and Bownes, Q. Rev. Biol. 65, 175-204 (1990); Bell et al., Cell 65, 229-239 (1991); Boggs et al., Cell 50, 739-747 (1987) and Burtis and Baker , the sex-determining lethal gene (Sxl, two separate transgenic lines-sgRNA Sxl , sgRNA Sxl-B ), transforming gene (tra, two separate lines-sgRNA Tra , sgRNA Tra-B ) or bisex genes (dsxF, sgRNA DsxF ), the entire contents of all documents are incorporated herein by reference. To disrupt male fertility, target genes that are active d...
Embodiment 2
[0101] Example 2. Create populations of up to 100% sterile males. In some embodiments of the present disclosure, the disclosed pgSIT methods can be used to disrupt genes essential for female viability and / or male sterility. In some embodiments, the disclosed pgSIT method can be used to concurrently or simultaneously disrupt genes necessary for female viability and male sterility to convert most or all (up to 100%) of surviving F 1 Offspring are genetically directed to sterile males. To achieve this directed characterization and sterility, three additional homozygous lines expressing multiple double gRNA (dgRNA) combinations including dgRNA βTub,Sxl , dgRNA βTub,Tra and dgRNA βTub,DsxF ,like Figure 1C shown in . To genetically assess the activity of these pgSIT lines, each line was bidirectionally crossed with wild-type or homozygous Cas9 (nos-Cas9, vas-Cas9, or Ubi-Cas9). refer to Figure 2A-2B , wild-type crosses did not produce significant sex bias or impaired ferti...
Embodiment 3
[0104] Example 3. Full penetrance due to zygotic expression . Maternal deposition of the Cas9 / gRNA complex in developing embryos is sufficient to ensure non-Mendelian inheritance of mutations in receiving offspring, even if those offspring do not genetically inherit the genes encoding the editing components. This phenomenon is known as the dominant maternal effect, as described in Lin and Potter, G3 (2016) doi: 10.1534 / g3.116.034884, the entire contents of which are incorporated herein by reference. In this regard, paternal inheritance of one of the core components (e.g., Cas9 or dgRNA) combined with maternal deposition of compatible components was investigated to determine whether either was sufficient to generate heritable mutations. refer to Figure 1G and 3B , mating between a homozygous Cas9 father and a heterozygous dgRNA-expressing mother was insufficient in F who did not inherit the dgRNA as a gene 1 Mutations (n=12) or knockout phenotypes (N=6, n=252) were induce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com