High-flux integrated phosphorylated proteomics detection method
A phosphorylation and analysis method technology, applied in the field of proteomics, can solve the problems of limited application, large amount of initial protein and other materials, etc., to achieve the effect of improving throughput and reducing sample analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
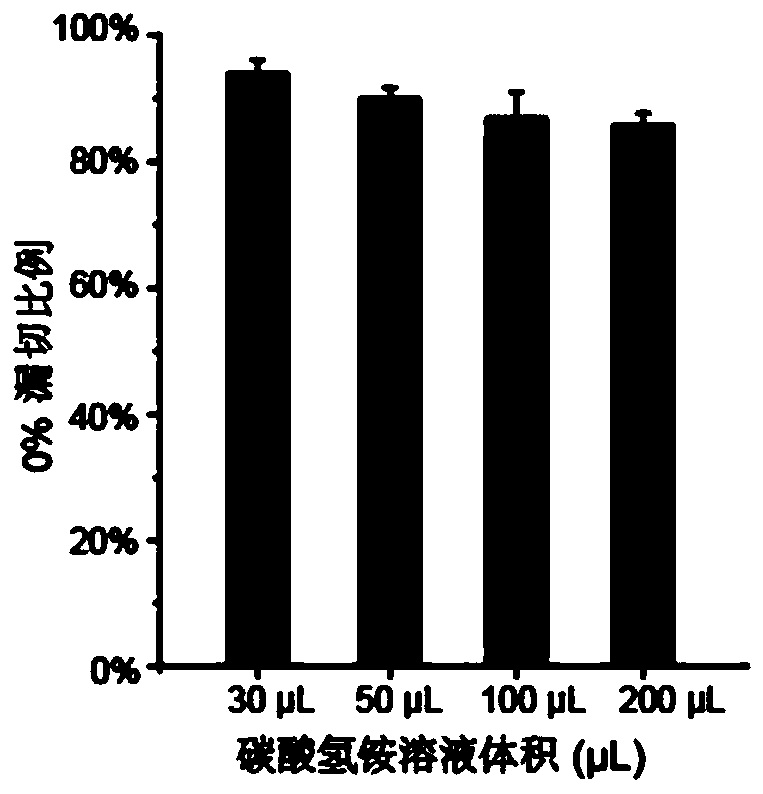
[0050] The optimization of different ammonium bicarbonate solution volumes to enzymolysis efficiency of embodiment 1
[0051] This example aims to realize the capture and analysis of related tyrosine phosphorylated proteins in a small amount of cell lysate, reduce the concentration of cell lysate, and test the feasibility and sensitivity of the method. The overall operation process is as follows figure 1 .
[0052] Add the corresponding amount of probe to the bait protein SH2 domain (1 mg / mL, dissolved in HEPES buffer) at a molar ratio of 1:10 (protein:probe), and incubate at room temperature for 2 minutes. Subsequently, 1 mol / L glycine solution was added according to the molar ratio of 1:10 (probe: glycine) to terminate the reaction between the probe and the bait protein. The resulting probe-labeled bait protein was ultrafiltered 7 times to remove unreacted probe and glycine molecules. Determine the protein concentration and dilute to 0.02 μg / μL. The bait protein labeled ...
Embodiment 2
[0054] Example 2 Exploration of the sensitivity of different cell dosages to sample pretreatment methods
[0055] According to the method in Example 1, 5 μg, 50 μg, and 300 μg of HeLa cell lysate before and after stimulation with EGF were added to the streptavidin-coated 96-well plate to which the probe and bait protein were connected (including 3 technical repetitions). ), and then carry out subsequent processing according to the general conditions described in Example 1, and carry out the comparison of protein complex enrichment and identification under EGF stimulation conditions.
[0056] The result is as Figure 3A As shown, as the amount of cell lysate increases, more protein complexes are enriched and identified after EGF stimulation. Among them, 17 EGFR signaling pathway-related proteins were enriched in only 5 μg of cell lysate. When the amount of cell lysate increased to 50 μg and 300 μg, 68 and 92 stimulation-related proteins could be enriched after EGF stimulation,...
Embodiment 3
[0058] Embodiment 3 method comparison
[0059] According to the method determined in Example 1 and Example 2, add 50 μg of HeLa cell lysate before and after EGF stimulation to the 96-well plate coated with streptavidin that has been connected to the probe and bait protein (including 3 times of technology) Repeat), then carry out follow-up treatment by general condition described in embodiment 1. On the other hand, in a 1.5mL centrifuge tube, add the same mass of bait protein linked to the probe, according to the patent 201910599017.6 treatment method, add 50μg of HeLa cell lysate before and after EGF stimulation (including 3 technical repetitions), and use 10μL streptavidin Avidin beads were used for capture, followed by subsequent processing according to the general conditions described in the patent 201910599017.6. The samples treated by the two methods were compared for the enrichment and identification of protein complexes under EGF stimulation conditions.
[0060] The r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com