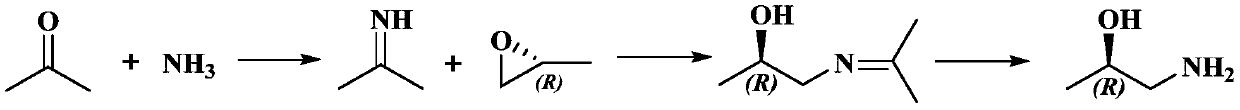Preparation method of chiral 1-amino-2-propanol
A chiral and amino technology, which is applied in the preparation of amino hydroxyl compounds, organic compounds, organic chemical methods, etc., can solve the problems of unfavorable environmental protection, waste acid, waste alkali and waste gas, and achieve convenient operation, high purity and Yield, the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
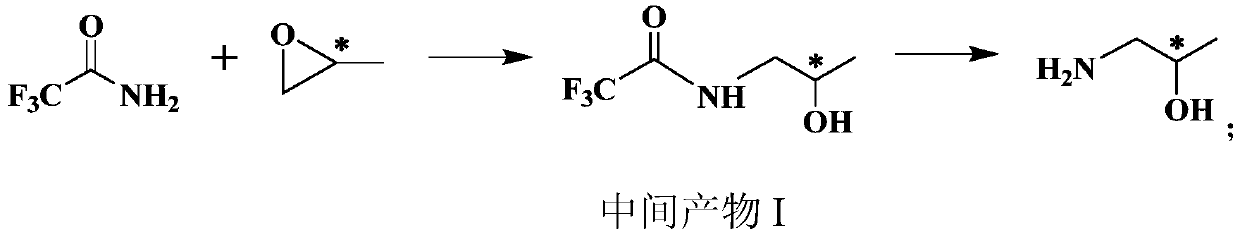
[0053] A preparation method of (S)-1-amino-2-propanol, which specifically includes the following steps:
[0054] (1)
[0055] Dissolve sodium tert-butoxide (230g, 2.39mol) in tetrahydrofuran (2L), slowly add trifluoroacetamide (226g, 2mol) under ice bath, stir for 30min; then add (S)-propylene oxide under ice bath (128g, 2.2mol), naturally warm up to room temperature, continue to stir the reaction for 10h, and then stir for another 2h at 35℃; after the reaction, add 1L of 2N (equivalent concentration) hydrochloric acid to the system for neutralization, and then add 1L of water. After standing for separation, the aqueous phase was extracted with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 318 g of intermediate product I with a yield of 93%.
[0056] Intermediate I 1 H-NMR(CDCl 3 , 400MHz): δ 1.08 (d, 3H), 3.37 (m, 2H), 4.0 (m, 1H), 7.12 (brs, 1H).
[0057] (2)
[0058] Dissolve the intermedi...
Embodiment 2
[0061] A method for preparing (R)-1-amino-2-propanol, specifically including the following steps:
[0062] (1)
[0063] Dissolve sodium tert-butoxide (300g, 3.12mol) in tetrahydrofuran (2L), slowly add trifluoroacetamide (294g, 2.6mol) under ice bath, stir for 30min; then add (R)-epoxy under ice bath Propane (166g, 2.96mol), naturally warm to room temperature, continue to stir and react for 10h, and then stir for 2h at 35℃; after the reaction, add 2N (equivalent concentration) hydrochloric acid to the system for neutralization, and then add water 1.3L, stand for layering, extract the aqueous phase with dichloromethane, combine the organic phases, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 423 g of intermediate product I with a yield of 95%.
[0064] Intermediate I 1 H-NMR(CDCl 3 , 400MHz): δ 1.07 (d, 3H), 3.36 (m, 2H), 3.99 (m, 1H), 7.11 (brs, 1H).
[0065] (2)
[0066] Dissolve the intermediate product I (420g, 2.45mol) obtained in step (1) ...
Embodiment 3
[0069] A method for preparing (R)-1-amino-2-propanol, specifically including the following steps:
[0070] (1)
[0071] Dissolve potassium tert-butoxide (303g, 2.7mol) in N,N-dimethylformamide (2L), slowly add trifluoroacetamide (282.5g, 2.5mol) under ice bath, and stir for 30min; Add (R)-propylene oxide (147.9g, 2.55mol) under the bath, naturally warm to room temperature, continue to stir and react for 6.5h, and then stir for another 1.5h at 35°C; after the reaction, add 2N (equivalent concentration) to the system. Neutralize with 0.8L of hydrochloric acid, then add 0.8L of water, stand to separate the layers, extract the aqueous phase with dichloromethane, combine the organic phases, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 398g of intermediate product I. The rate is 93%.
[0072] (2)
[0073] Intermediate product I (390g, 2.28mol) obtained in step (1) was dissolved in methanol (1.5L), 350mL of water and sodium carbonate (477g, 4.5mol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com