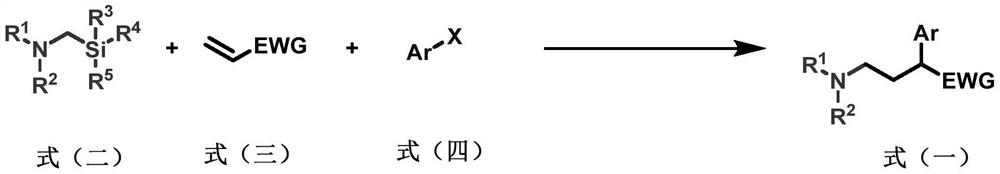A kind of preparation method of 2-aryl-γ-aminobutyric acid derivative
A technology of aminobutyric acid and its derivatives, which is applied in the field of preparation of 2-aryl-γ-aminobutyric acid derivatives, can solve the problems of cumbersome steps, harsh reaction conditions, poor compatibility of reactive functional groups, etc., and achieve good reaction selectivity , mild reaction conditions, wide substrate universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
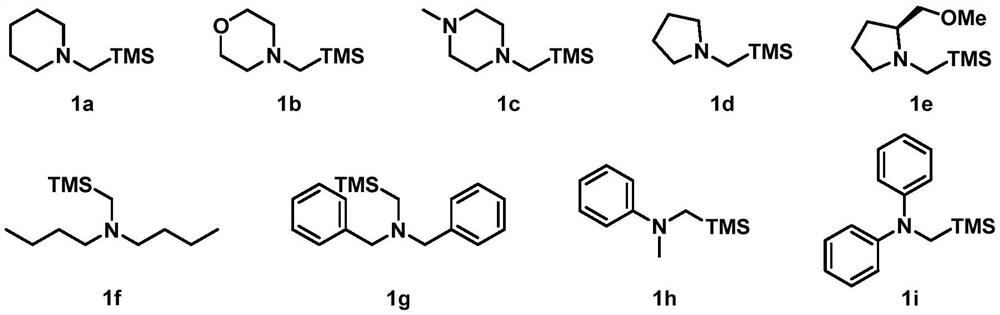
[0060] Add a magnetic stirrer in a 10mL transparent glass reaction tube, photocatalyst Ir(ppy) 2 (bpy)PF 6 (2.4mg, 3.0μmol, 0.010eq), metal catalyst NiCl 2 Glyme (6.6mg, 30μmol, 0.10equivalent), ligand di(OMe)bpy (6.1mg, 30μmol, 0.10equivalent), seal the nozzle with a rubber stopper, protect the reaction system with nitrogen gas (exhaust gas three times), inject the syringe into Solvent anhydrous DMF (3.0 mL), followed by addition of α-aminomethylsilyl 1a (0.12 mL, 0.10 g, 0.60 mmol, 2.0 equiv), benzyl acrylate 2a (90 μL, 97 mg, 0.60 mmol, 2.0 equiv) with a micro syringe , iodobenzene 3a (33μL, 61mg, 0.30mmol, 1.0eq), reacted at room temperature under blue light for 15 hours, then quenched the reaction with water, extracted with ethyl acetate (20mL x 3 times), combined the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave product 4 (78 mg, yield 77%). 1 H NMR (400MHz, CDCl 3)δ:7.34–7.22(m,10H),5.13(d,J=12.5Hz,1H)...
Embodiment 2
[0062] Add a magnetic stirrer in a 10mL transparent glass reaction tube, photocatalyst Ir(ppy) 2 (bpy)PF 6 (2.4mg, 3.0μmol, 0.010eq), metal catalyst NiCl 2 Glyme (6.6mg, 30μmol, 0.10equivalent), ligand di(OMe)bpy (6.1mg, 30μmol, 0.10equivalent), seal the nozzle with a rubber stopper, protect the reaction system with nitrogen gas (exhaust three times), inject the syringe into Solvent Anhydrous DMF (3.0 mL), followed by microsyringe addition of α-aminomethylsilyl 1b (0.10 g, 0.60 mmol, 0.60 mmol, 2.0 equiv), benzyl acrylate 2a (90 μL, 97 mg, 0.60 mmol, 2.0 equiv) , iodobenzene 3a (33μL, 61mg, 0.30mmol, 1.0eq), reacted at room temperature under blue light for 15 hours, then quenched the reaction with water, extracted with ethyl acetate (20mL x 3 times), combined the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave product 5 (90 mg, yield 88%). 1 H NMR (400MHz, CDCl 3 )δ:7.37–7.22(m,10H),5.14(d,J=12.4Hz,1H),5.06(d,J...
Embodiment 3
[0064] Add a magnetic stirrer in a 10mL transparent glass reaction tube, photocatalyst Ir(ppy) 2 (bpy)PF 6 (2.4mg, 3.0μmol, 0.010eq), metal catalyst NiCl 2 Glyme (6.6mg, 30μmol, 0.10equivalent), ligand di(OMe)bpy (6.1mg, 30μmol, 0.10equivalent), seal the nozzle with a rubber stopper, protect the reaction system with nitrogen gas (exhaust three times), inject the syringe into Solvent anhydrous DMF (3.0 mL), followed by addition of α-aminomethylsilyl 1c (0.11 g, 0.60 mmol, 0.60 mmol, 2.0 equiv), benzyl acrylate 2a (90 μL, 97 mg, 0.60 mmol, 2.0 equiv) with a micro syringe , iodobenzene 3a (33μL, 61mg, 0.30mmol, 1.0eq), reacted at room temperature under blue light for 15 hours, then quenched the reaction with water, extracted with ethyl acetate (20mL x 3 times), combined the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave product 6 (95 mg, yield 90%). 1 H NMR (400MHz, CDCl 3 )δ:7.34–7.27(m,8H),7.26–7.22(m,2H),5.14(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com