Recombinant escherichia coli for synthesizing lactoyl N-neotetraose and construction method and application of recombinant escherichia coli
A technology for recombining Escherichia coli and Escherichia coli, applied in the field of metabolic engineering, can solve the problems of multi-flow of carbon sources, insufficient stability of plasmid expression, regulation of metabolic pathways, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
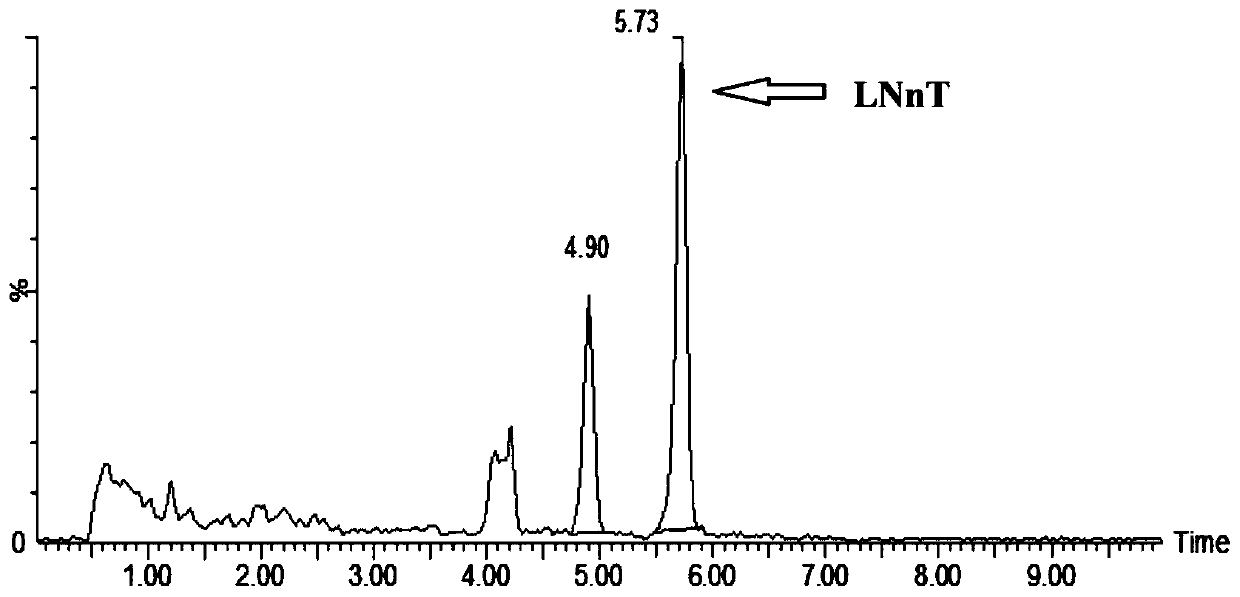
Image
Examples
Embodiment 1
[0028] Example 1: Construction of Gene Knockout Homology Arm Fragments
[0029] According to the sequence information of E.coli MG1655, the primers shown in Table 1 were designed. The integration site of the lacY gene was fliK. Using the above primers, the E.coli MG1655 genome was used as a template to amplify lacZ, nagB, wecB, ugd, The upstream and downstream homology arm fragments of each gene of fliK were respectively fused by Overlapping PCR to obtain fragments lacZ12, nagB12, wecB12, ugd12, and lacY12.
[0030] Table 1
[0031]
Embodiment 2
[0032] Embodiment 2: Construction of pTarget plasmid
[0033] Select the N20 region (20bp base sequence used to target the gene to be knocked out in the CRISPR / Cas9 system) on each gene to be knocked out, design primers, use pTarget as a template, and replace the N20 region of the template by PCR. The PCR product was transferred into E.coli JM109, and the plasmid was extracted to obtain 4 plasmids, pTarget-lacZ, pTarget-nagB, pTarget-wecB, pTarget-ugd, pTarget-lacY.
Embodiment 3
[0034] Example 3: Gene knockout and integration
[0035] The starting strain E.coli MG1655 was recorded as M0, and the pCas9 plasmid was transformed into M0 to obtain the host M0 (pCas9) expressing the Cas9 protein, and then the pTarget-lacZ plasmid and fragment lacZ12 were electrotransformed into M0 (pCas9), and the lacZ gene was knocked out. M01(pCas9), eliminate the pTarget-lacZ plasmid, electrotransfer the pTarget-nagB plasmid and the fragment nagB12 into M01(Cas9), knock out the nagB gene, knock out each gene to be knocked out in this way, and finally eliminate the pTarget and pCas9 plasmids , and M04 with lacZ, nagB, wecB, and ugd gene knockouts was obtained.
[0036] On the basis of the M04 strain that knocked out the lacZ, nagB, wecB, and ugd genes, the pCas9 plasmid was transformed into M04 to obtain the host M04 (pCas9) expressing the Cas9 protein, and then the pTarget-lacY plasmid and the lacY gene, tac promoter , the fusion fragment lacY12 of the upper and lower h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com