A kind of artemisinin-piperazine-furanone derivative and its preparation method and application
A derivative, artemisinone technology, applied in the field of artemisinone-piperazine-furanone derivatives and its preparation, can solve the problems of poor therapeutic effect and high clinical recurrence rate, achieve simple operation and improve anti-tumor activity , the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Artemisinin-piperazine-furanone derivatives
[0056] The structural formula of the above-mentioned artemisinin-piperazine-furanone derivatives is shown in formula 1:
[0057]
[0058] 2. Preparation method
[0059] The preparation method of above-mentioned artemisinin-piperazine-furanone derivatives comprises the following steps:
[0060] (a) Artemisinone-piperazine and 3,4-dichloro-5-methoxyfuranone are dissolved in acetonitrile to obtain a mixture, wherein the molar ratio of artemisinone-piperazine to halogenated furanone is 1 : 1, the concentration of artesunate-piperazine in the mixture is 0.012mmol / mL;
[0061] (b) Diisopropylethylamine is added to the mixture, and the reaction is carried out under stirring conditions, wherein the molar ratio of diisopropylethylamine to artesunone-piperazine is 3: 1, and the reaction temperature is room temperature, The reaction time is 4h;
[0062] (c) After the reaction is completed, the solvent is evaporated, and the m...
Embodiment 2
[0070] 1. Artemisinin-piperazine-furanone derivatives
[0071] The structural formula of the above-mentioned artemisinin-piperazine-furanone derivatives is shown in formula 2:
[0072]
[0073] 2. Preparation method
[0074] The preparation method of the above artesunone-piperazine-furanone derivatives is basically the same as the preparation method in Example 1, the only difference is that 3,4-dichloro-5-menthyloxyfuranone is replaced by 3,4- Dichloro-5-methoxyfuranone.
[0075] Weigh the mass of artesunone-piperazine-furanone derivatives prepared above, and then calculate the weighed mass÷theoretical yield×100%, and the yield of the above preparation method is 82%.
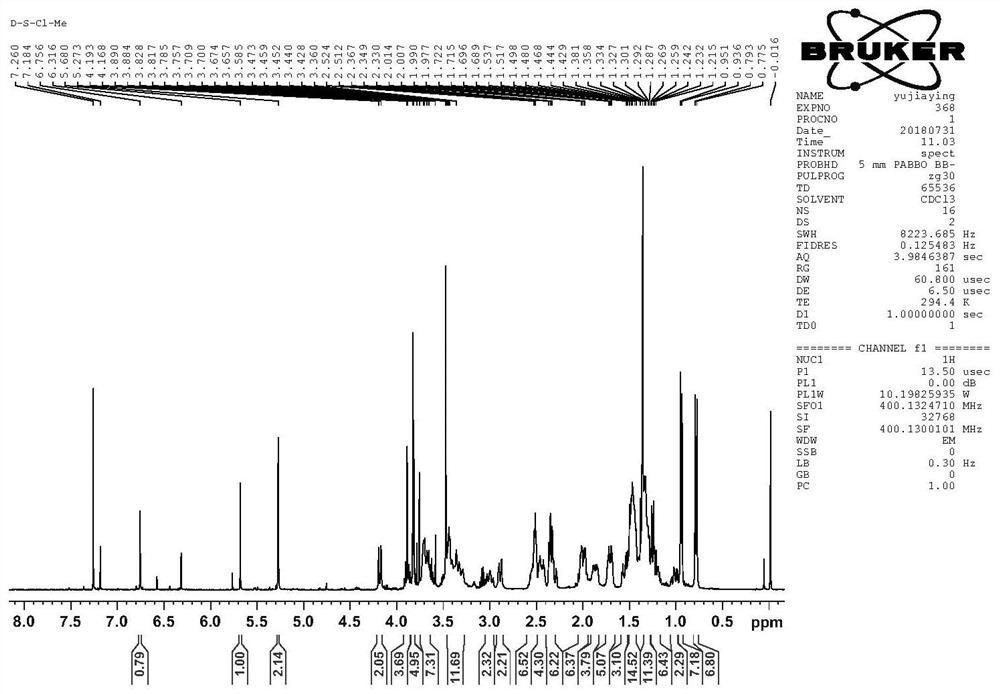
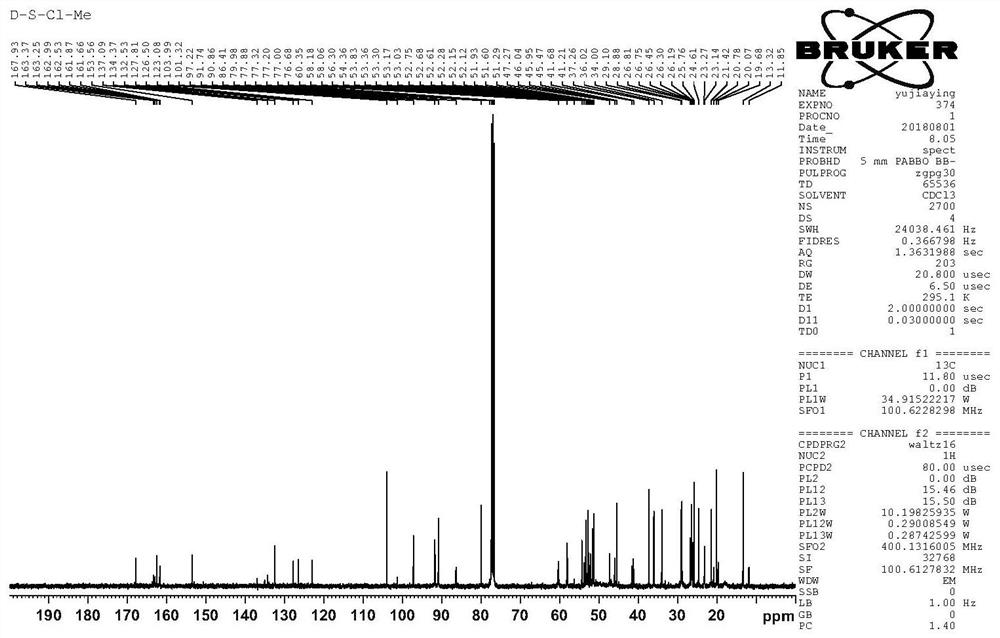
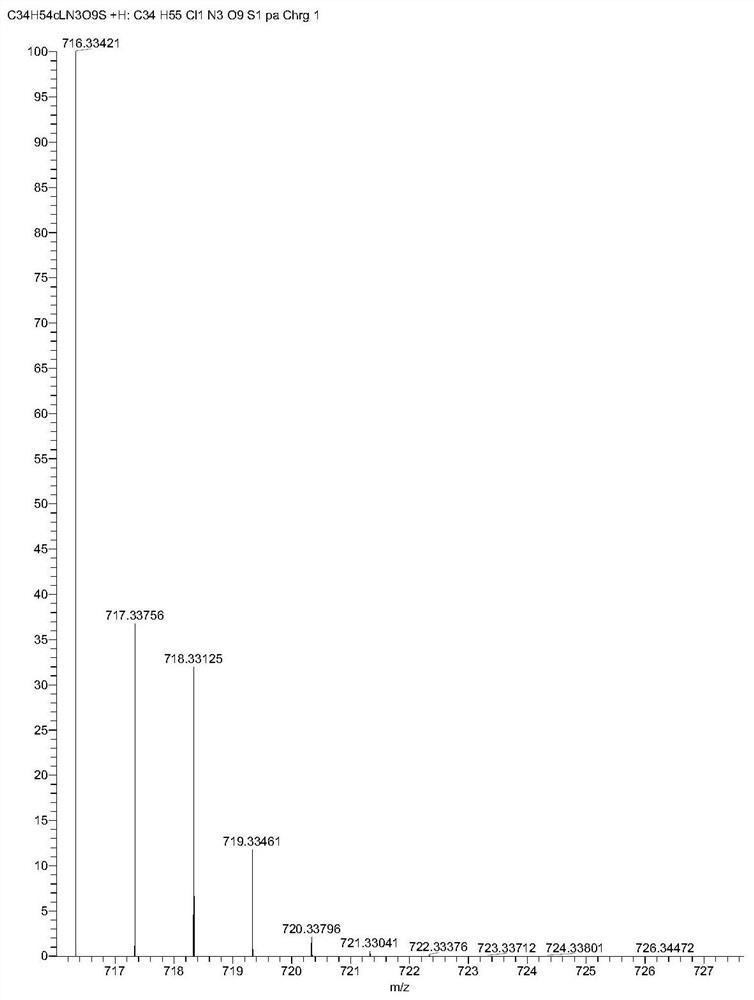
[0076] The artesunone-piperazine-furanone derivatives prepared above were selected for nuclear magnetic resonance and mass spectrometry analysis; 1 H NMR spectrum, 13 C NMR spectrum and mass spectrogram as Figure 4-6 shown;
[0077] Depend on Figure 4-6 It can be seen that: 1 H NMR (CDCl 3 )δ5.78(d...
Embodiment 3
[0082] 1. Artemisinin-piperazine-furanone derivatives
[0083] The structural formula of the above-mentioned artemisinin-piperazine-furanone derivatives is shown in formula 3:
[0084]
[0085] 2. Preparation method
[0086] The preparation method of the above artesunone-piperazine-furanone derivatives is basically the same as the preparation method in Example 1, the only difference is that 3,4-dichloro-5-bornyloxyfuranone is replaced by 3,4- Dichloro-5-methoxyfuranone.
[0087] Weigh the mass of artesunone-piperazine-furanone derivatives prepared above, and then calculate the weighed mass÷theoretical yield×100%, and the yield of the above preparation method is 80%.
[0088] The artesunone-piperazine-furanone derivatives prepared above were selected for nuclear magnetic resonance and mass spectrometry analysis; 1 H NMR spectrum, 13 C NMR spectrum and mass spectrogram as Figure 7-9 shown;
[0089] Depend on Figure 7-9 It can be seen that: 1 H NMR (CDCl 3 )δ5.73(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com