Cyclodextrin glucosyltransferase mutants with improved disproportionation specific activity and aa-2g production
A glucose-based and cyclodextrin technology, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as low conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of cyclodextrin glucosyltransferase single mutant recombinant bacteria
[0034] (1) Single mutation of cyclodextrin glucosyltransferase
[0035] According to the nucleotide sequence of the cyclodextrin glucosyltransferase shown in the amino acid sequence position SEQ ID NO. 2, the primers with Y195F and Y260F mutations were designed and synthesized, and the cyclodextrin glucosyltransferase gene was site-directed mutagenesis, Single mutants mutated at positions 195 and 260 were obtained, respectively.
[0036] Using rapid PCR technology, using the expression vector pET20b(+) / cgt as a template (the vector already contains a promoter and a signal peptide, and the sequence of the target gene after the signal peptide), the construction method of the expression vector pET20b(+) / cgt is described in Li Zhaofeng, "The expression of Paenibacillus softening α-cyclodextrin glucosyltransferase in Escherichia coli and its product specificity analysis" (publish...
Embodiment 2
[0056] Example 2: Preparation of cyclodextrin glucosyltransferase double mutant recombinant bacteria
[0057] On the basis of the single mutant Y195F, the double mutant was constructed by using the site-directed mutagenesis primer of Y260F mutation. For specific embodiments, see Example 1, using the rapid PCR technology, using the recombinant plasmid cgt / pET20b(+) as a template, the construction method of the recombinant plasmid cgt / pET20b(+) is described in Li Zhaofeng's "Paenibacillus softening α-cyclodextrin glucose" Expression of basal transferase in Escherichia coli and analysis of its product specificity" (published in 2009).
[0058] The site-directed mutagenesis primers for introducing the Y260F mutation are:
[0059] Forward primer: CGGGGAATGG TTC CTTGGCCGCGGATCAAAC, SEQ ID NO. 8,
[0060] Reverse primer: GTTTGATCCGCGCCAAG GAA CCATTCCCCG, SEQ ID NO. 9.
[0061] For the preparation method of the recombinant bacteria, see Example 1, and the recombinant bacteria ob...
Embodiment 3
[0062] Example 3: Fermentation of mutant recombinant bacteria to produce enzymes
[0063] The recombinant strains obtained in Examples 1 and 2 were picked and grown for 8-10 h in LB liquid medium (containing 100 μg / mL ampicillin), and the seed fermentation broth was connected to TB medium (containing 100 μg / mL ampicillin by 5% inoculum size). ), after culturing in a shaker at 25 °C for 60 h, the fermentation broth was centrifuged at 4 °C and 8000 rpm for 10 min to remove bacteria, and the centrifuged supernatant was collected to obtain crude enzyme liquid.
[0064] The E. coli BL21 (DE3) containing the wild-type enzyme was fermented to produce the enzyme in the same way.
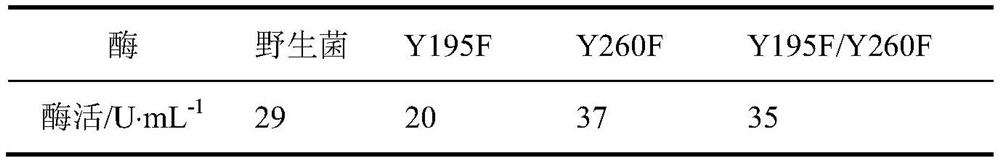
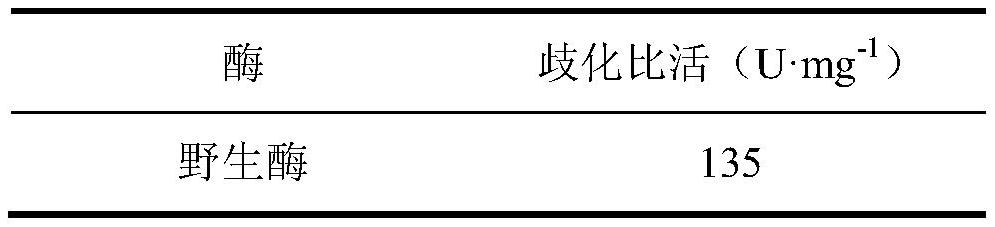
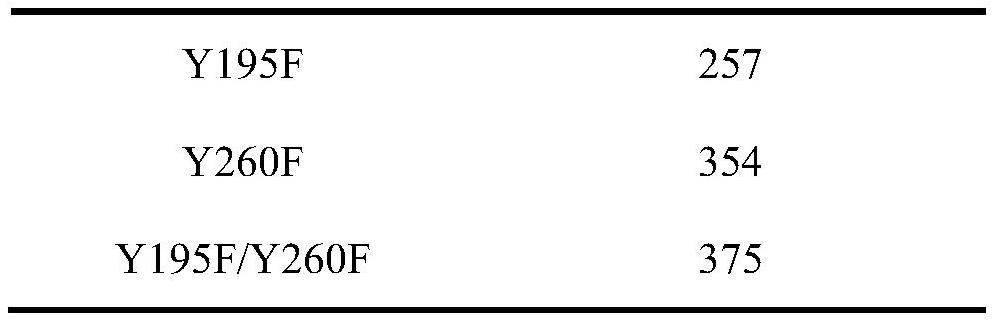
[0065] The enzymatic activities of wild-type, Y195F, Y260F, Y195F / Y260F were measured respectively. The results are shown in Table 1. The enzymatic activity of mutant Y195F was slightly lower than that of wild-type, and the enzymatic activities of mutant Y260F and Y195F / Y260F were increased compared with wil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com