A kind of method for preparing Belite sulfoaluminate cement co-producing sulfuric acid
A belite sulfoaluminate and special sulfoaluminate technology, applied in the field of building materials and chemical products, can solve the problem of high operating cost, inability to effectively utilize industrial waste slag gypsum on a large scale, and no co-production process of sulfuric acid has been found. technical or methodological issues to achieve the effect of saving resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
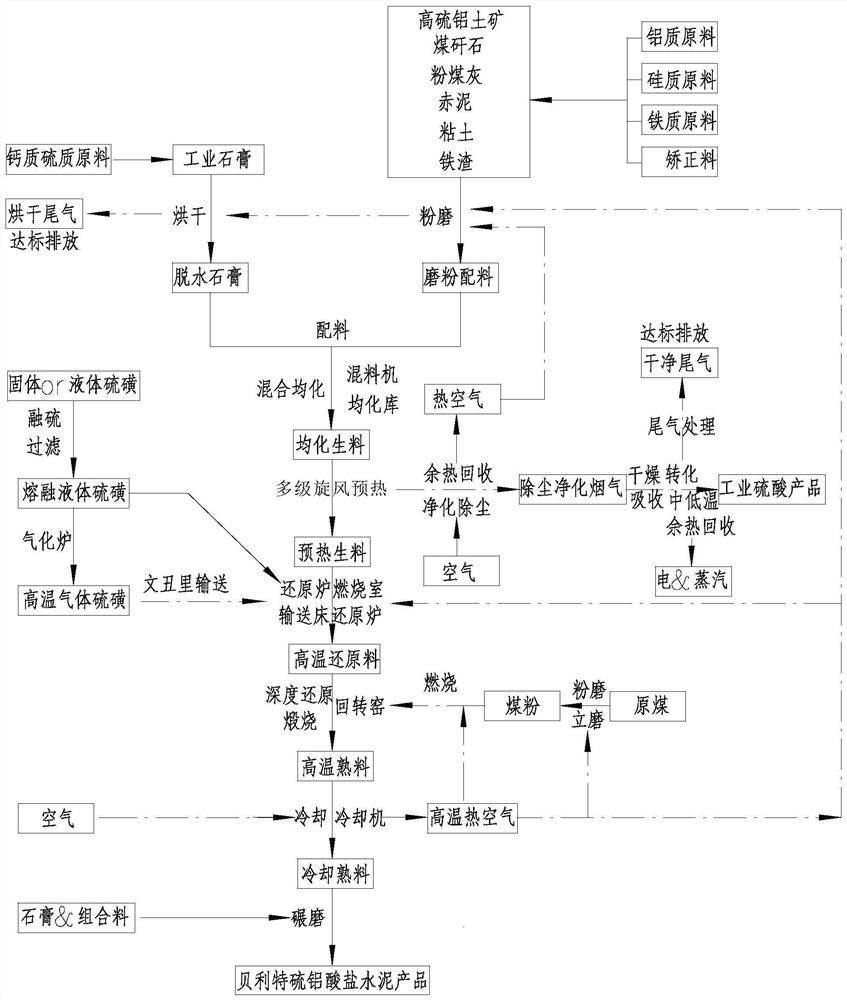
[0076] Process such as figure 2 As shown in the figure, phosphogypsum, high-sulfur bauxite and fly ash are used as raw materials, and the raw materials are dried and pulverized to > 95%-80 μm. The chemical composition of raw materials and raw meal is shown in Table 1.
[0077] Table 1 Chemical composition of raw materials and raw meal
[0078] project CaO SiO 2
Al 2 O 3
Fe 2 O 3
MgO P 2 O 5
Na 2 O
SO 3
LOSS total Phosphogypsum 32.56 4.76 0.97 0.39 0.31 1.05 0.30 43.63 15.98 99.95 High sulfur bauxite 2.42 22.55 52.93 7.45 0.09 0.00 0.21 6.25 7.52 99.42 fly ash 1.48 43.50 35.38 1.92 0.70 0.00 0.33 0.68 14.77 98.76 Raw material 27.31 9.47 8.65 1.19 0.32 0.87 0.29 36.68 15.10 100.00
[0079] The above-mentioned phosphogypsum dihydrate, high-sulfur bauxite, and fly ash raw materials are composed by weight: 82.7 parts of phosphogypsum dihydrate, 9.6 parts of ...
Embodiment 2
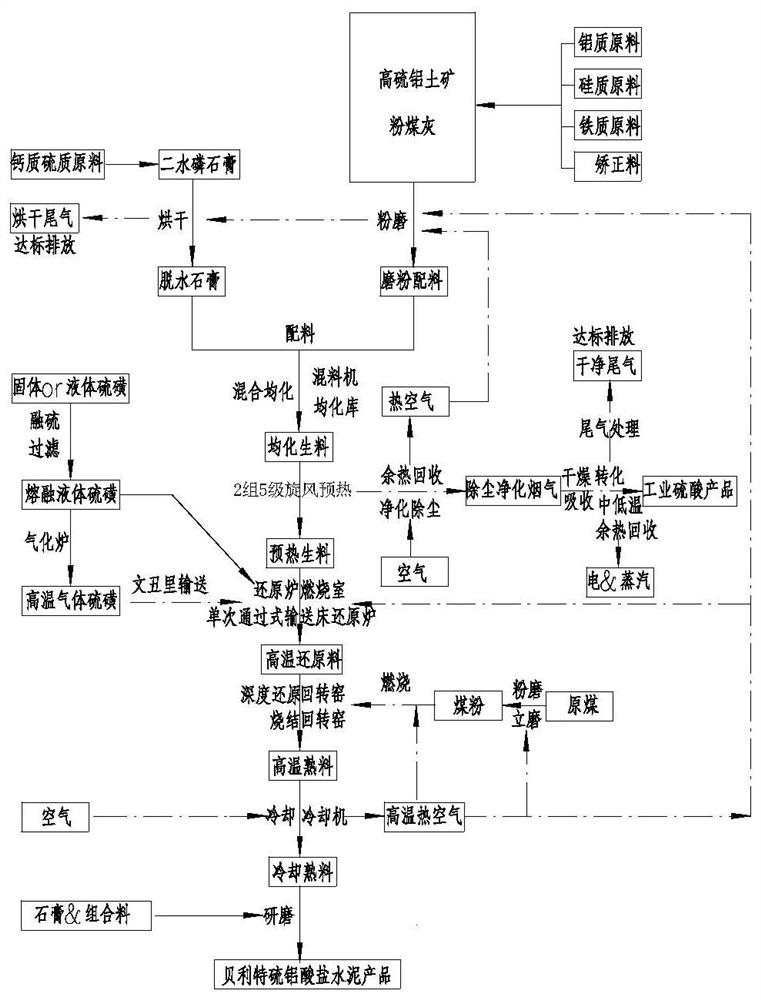
[0088] Process such as image 3 As shown, using dihydrate phosphogypsum and high-sulfur and low-grade bauxite as raw materials, the raw materials are dried and pulverized to > 95%-80 μm, and most of the fly ash produced by the combustion of fuel coal in the rotary kiln enters the clinker. The chemical composition of raw materials and raw meal is shown in Table 4.
[0089] Table 4 Chemical composition of raw materials and raw meal
[0090] project CaO SiO 2
Al 2 O 3
Fe 2 O 3
MgO P 2 O 5
Na 2 O
SO 3
LOSS total Phosphogypsum 32.56 4.76 0.97 0.39 0.31 1.05 0.30 43.63 15.98 99.95 High sulfur bauxite 2.42 20.35 44.18 12.86 0.09 0.00 0.21 11.37 7.82 99.30 Raw material 26.58 7.89 9.63 2.89 0.27 0.84 0.28 37.24 14.37 100.00
[0091] The above-mentioned phosphogypsum dihydrate and high-sulfur and low-grade bauxite raw materials are composed by weight as follows: 80.0 parts of ph...
Embodiment 3
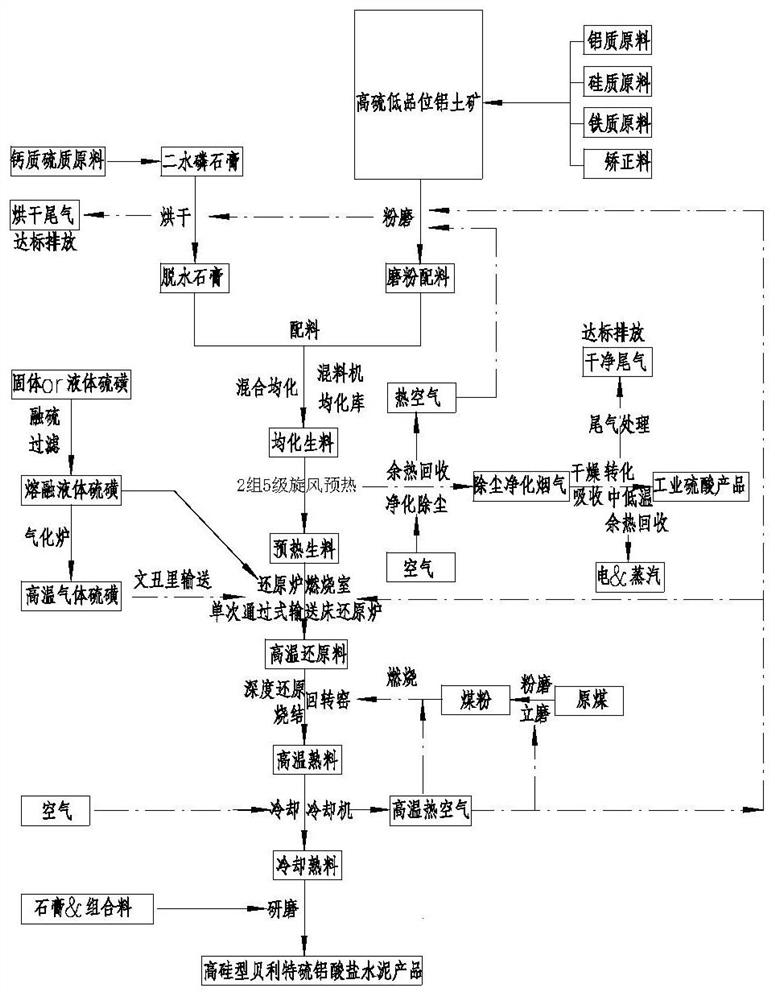
[0099] Process such as Figure 4 As shown, using desulfurized gypsum, high-sulfur and high-iron bauxite, and fly ash as raw materials, the raw materials are dried and pulverized to > 95%-80 μm, and most of the fly ash produced by the combustion of the fuel coal in the rotary kiln enters the clinker , and its chemical composition is shown in Table 7.
[0100] Table 7 Chemical composition of raw materials and raw meal
[0101] project CaO SiO 2
Al 2 O 3
Fe 2 O 3
MgO TiO 2
Na 2 O
SO 3
LOSS total Desulfurized gypsum 34.35 1.56 1.78 1.15 2.24 0.12 0.15 41.56 17.06 99.97 High sulfur bauxite 2.42 20.35 44.18 12.86 0.09 0.65 0.21 11.37 7.82 99.95 fly ash 1.48 43.50 35.38 1.92 0.70 1.18 0.33 0.68 14.77 99.94 Raw material 27.16 8.00 10.37 2.69 1.82 0.29 0.18 33.81 15.69 100.00
[0102] The above-mentioned dihydrate desulfurization gypsum, high-sulfur and low-grade b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com