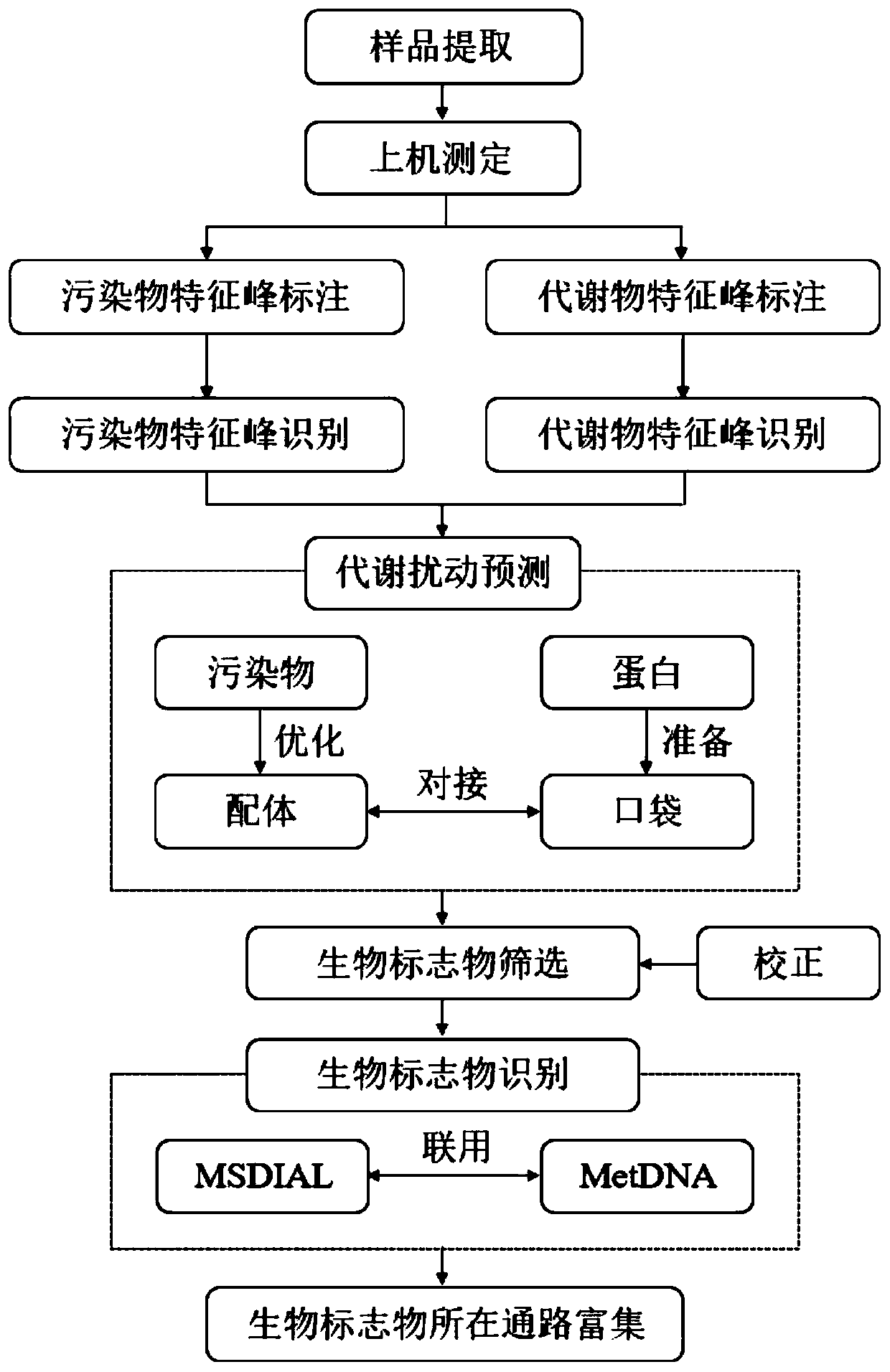
Non-target biomarker high-throughput screening method based on pollutant metabolism disturbance
A technology for biomarkers and pollutants, applied in measurement devices, instruments, scientific instruments, etc., can solve problems such as low accuracy and insufficient screening throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] This embodiment is a high-throughput screening method for non-target biomarkers of metabolic disturbance caused by perfluorinated pollutants in blood media, and the steps are as follows:
[0082] (1) Take 0.5mL serum sample in a 15mL centrifuge tube, add 0.26~0.28g of magnesium sulfate-sodium chloride mixture and 1.5mL of acetonitrile and vortex immediately, the sample is in a suspension state at this time, after the sample is ultrasonically extracted for 30min Centrifuge and transfer supernatant. The remaining residue was repeatedly extracted twice with 95% acetonitrile-water solution, and the extracts were combined. Nitrogen was blown to near dryness, transferred to a chromatographic vial and dilute to 100 μL with acetonitrile.
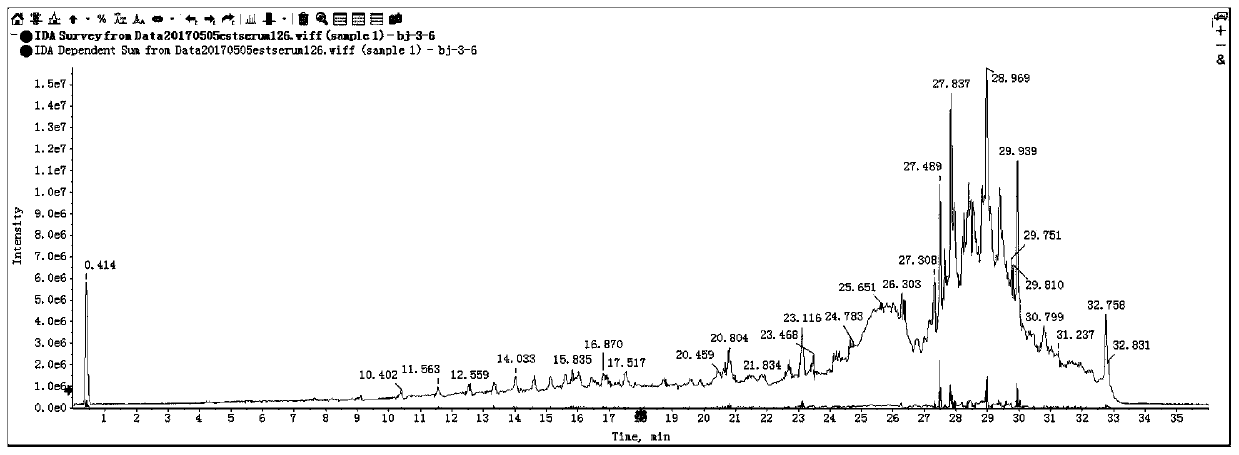
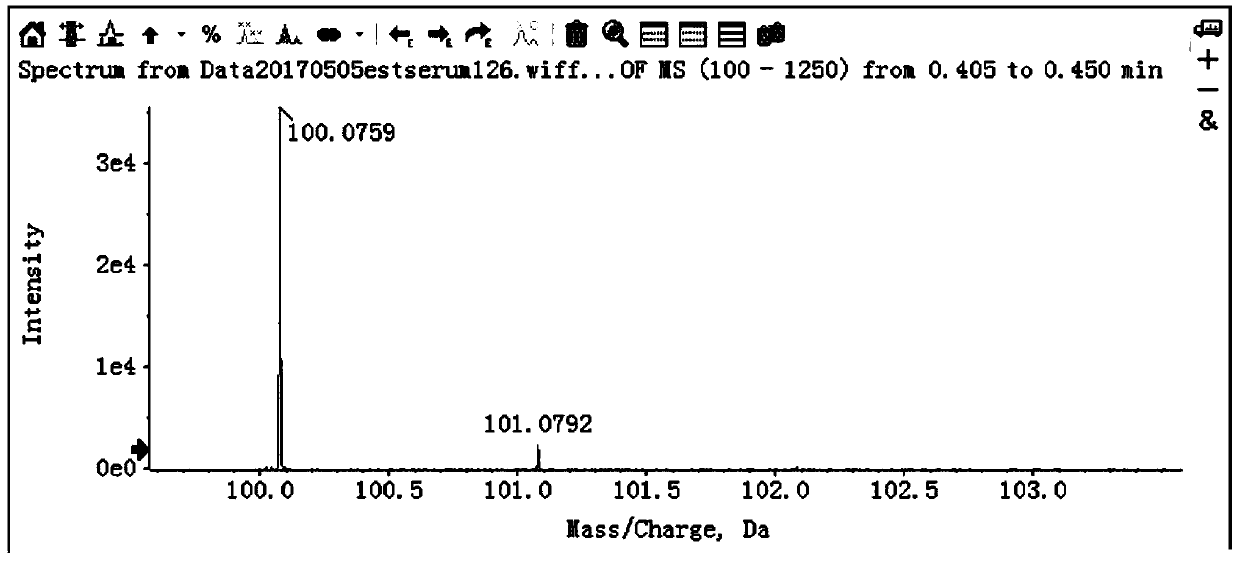
[0083] (2) On-board measurement: the extracted samples are analyzed and detected in full scan by high performance liquid chromatography-time-of-flight mass spectrometry. Its parameters are:
[0084] High performance liquid chromatography: ...
Embodiment 2
[0139] The operating steps of this embodiment are basically the same as those in Example 1, except that: before step (5), the two most widely studied perfluorinated compounds for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are added. Docking steps to make predictions about their metabolic perturbation capabilities.
[0140] The molecules of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) were optimized in SYBYL software: applying Tripos force field, Gasteiger-Huckel charge, and Powell (Powell) gradient method until the termination gradient drops to 0.001kcal / (mol· )the following.
[0141] After downloading the human serum albumin structure, the ligand was extracted in SYBYL software to form a docking pocket, and the crystal water was removed and protonated. The optimized perfluoroligand was docked with the protein pocket in SYBYL software, and the optimal conformation was selected as the docking result. The greater the total...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com