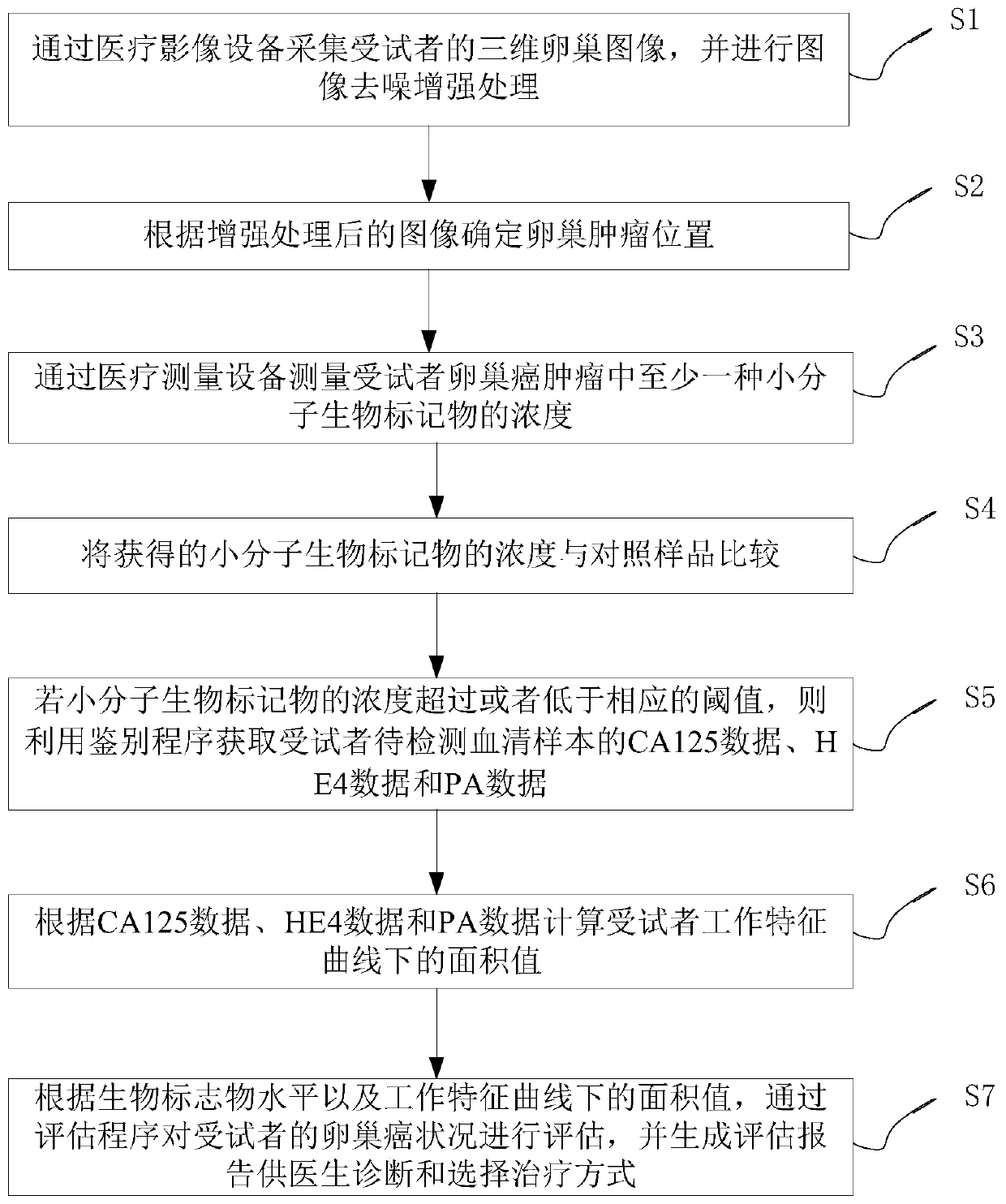
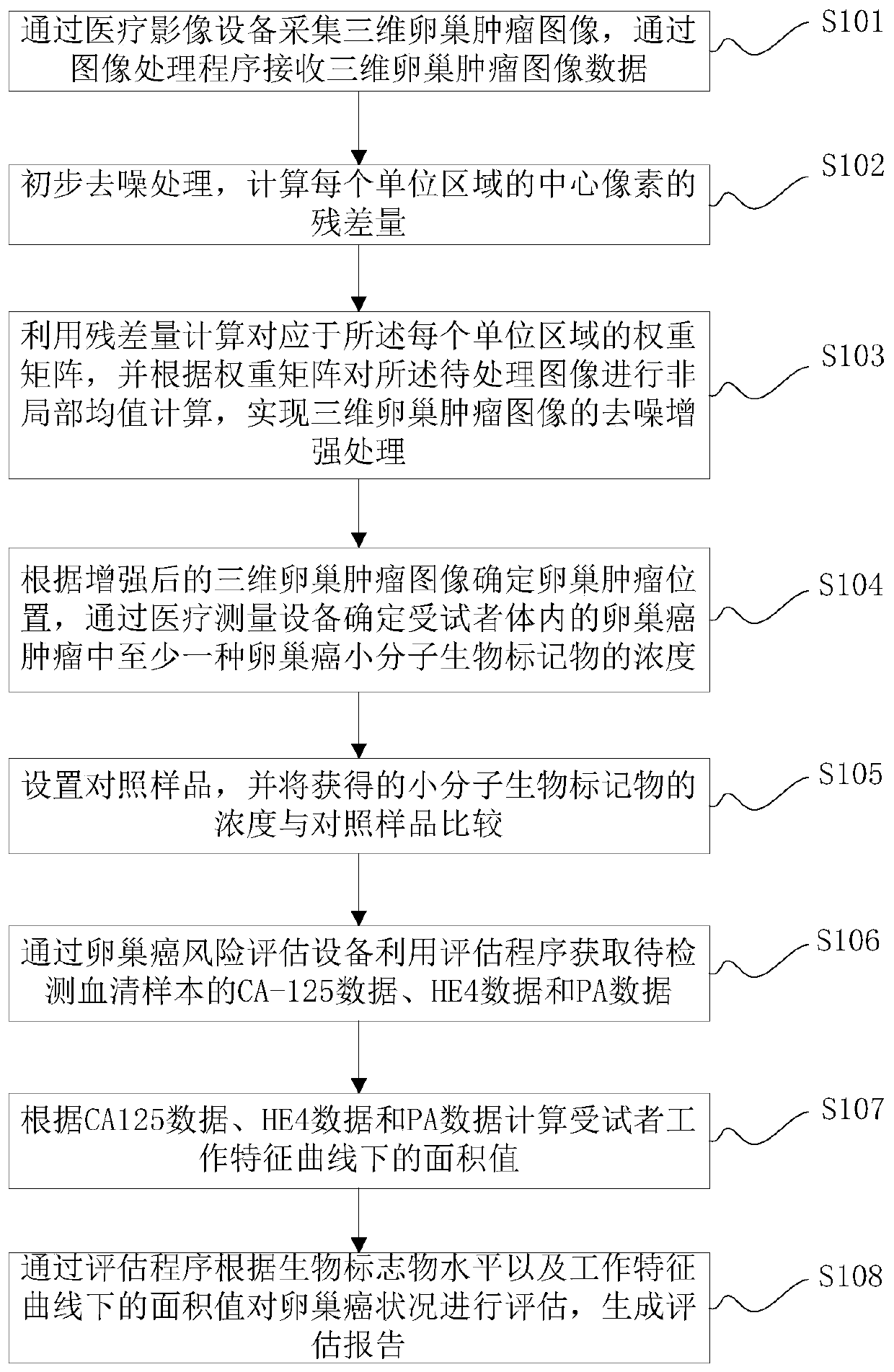
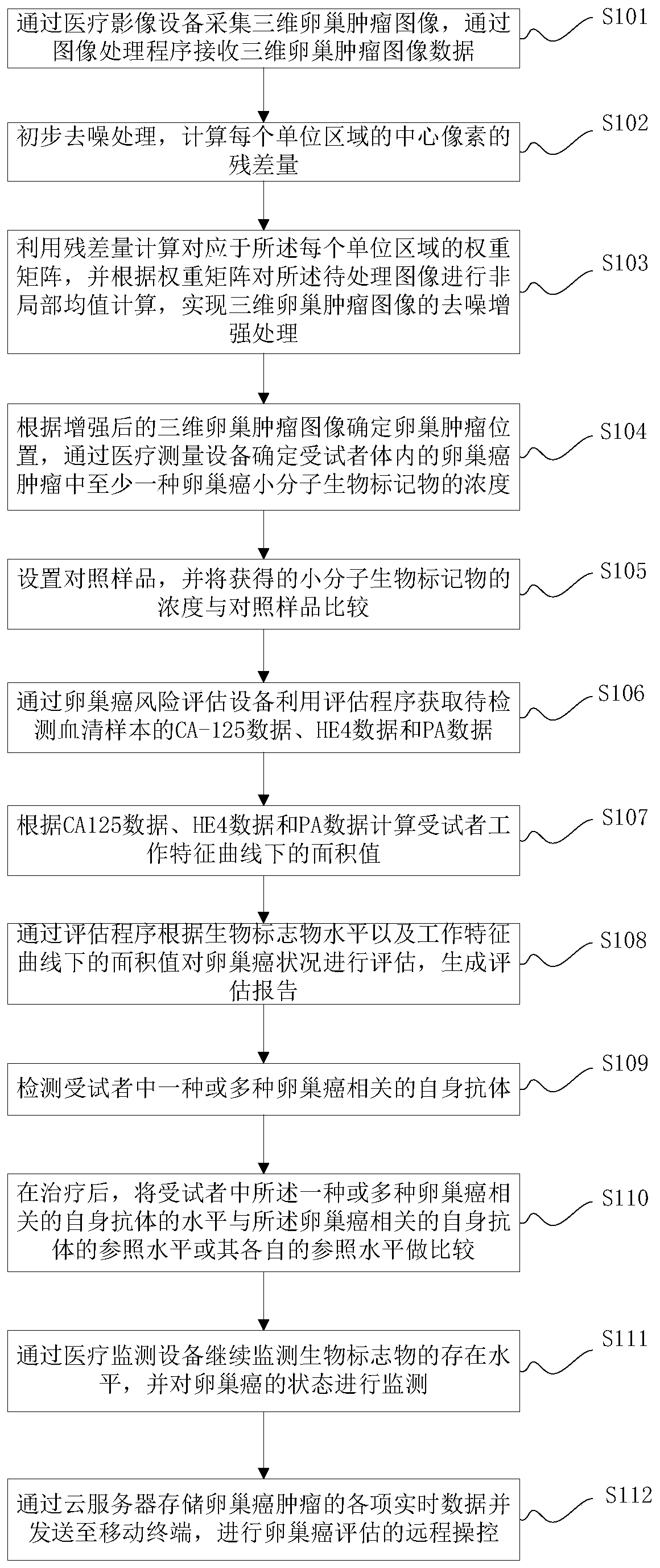
Application method and system of biomarker in evaluation of ovarian cancer
A biomarker and ovarian cancer technology, applied in the field of ovarian cancer assessment, can solve the problems of low sensitivity and specificity of early detection and early warning of ovarian cancer malignant tumors, imperfect evaluation of ovarian cancer treatment effect, etc., to achieve simplified measurement, The effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] 1. Experimental materials
[0148] Serum samples from patients with ovarian cancer were collected from patients undergoing ovarian cancer surgery at Renmin Hospital of Wuhan University during preoperative examinations. All patients had not received radiation therapy, chemotherapy and antineoplastic drugs before operation, and all had complete clinical and pathological data. 95 patients with ovarian cancer (ovarian cancer group), aged 34-66 years, with an average age of 53.3 years. Serum samples of 40 healthy volunteers (normal control group), aged 30-65 years, with an average age of 54.5 years. There was no significant difference in age composition between the ovarian cancer group and the normal control group.
[0149] 2. Experimental method
[0150] (1) Acquire the CA-125 data, HE4 data and PA data of the serum sample to be tested.
[0151] (2) Determine the area under the receiver operating characteristic curve according to the CA-125 data, the HE4 data and the PA...
Embodiment 2
[0164] The application method of the biomarkers provided in the embodiments of the present invention in the evaluation of ovarian cancer is as follows: figure 1 As shown, as a preferred embodiment, such as Figure 10 As shown, the method for measuring the concentration of ovarian cancer biomarkers in ovarian cancer tumors by medical measuring equipment provided by the embodiments of the present invention includes:
[0165] S201. Determine the concentration of at least one small molecule biomarker from a sample from the subject; wherein, when compared with a control sample, an increase or decrease in the concentration of the sample indicates that the subject is suffering from ovarian cancer, or have an increased risk of developing ovarian cancer.
[0166] S202. Compared with the control, the at least one small molecule biomarker whose concentration is increased is selected from the group consisting of dihydroxybutyric acid and trihydroxybutyric acid.
[0167] S203. Compared w...
Embodiment 3
[0176] The application method of the biomarkers provided in the embodiments of the present invention in the evaluation of ovarian cancer is as follows: figure 1 As shown, as a preferred embodiment, such as Figure 11 As shown, the method for identifying benign and malignant ovarian cancer by using the identification program provided by the ovarian cancer identification device provided in the embodiment of the present invention includes:
[0177] S301. Acquire CA-125 data, HE4 data and PA data of the serum sample to be tested.
[0178] S302. Determine an area value under a receiver operating characteristic curve according to the CA-125 data, the HE4 data, and the PA data.
[0179] S303. Determine whether the ovarian mass corresponding to the serum sample to be detected is good or bad according to the area value.
[0180] The determination of the area value under the receiver operating characteristic curve according to the CA-125 data, the HE4 data and the PA data provided in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com