Novel E3 ubiquitin ligase TcpC and application thereof
A technology of ubiquitin ligase and protein, applied in the fields of molecular biotechnology and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
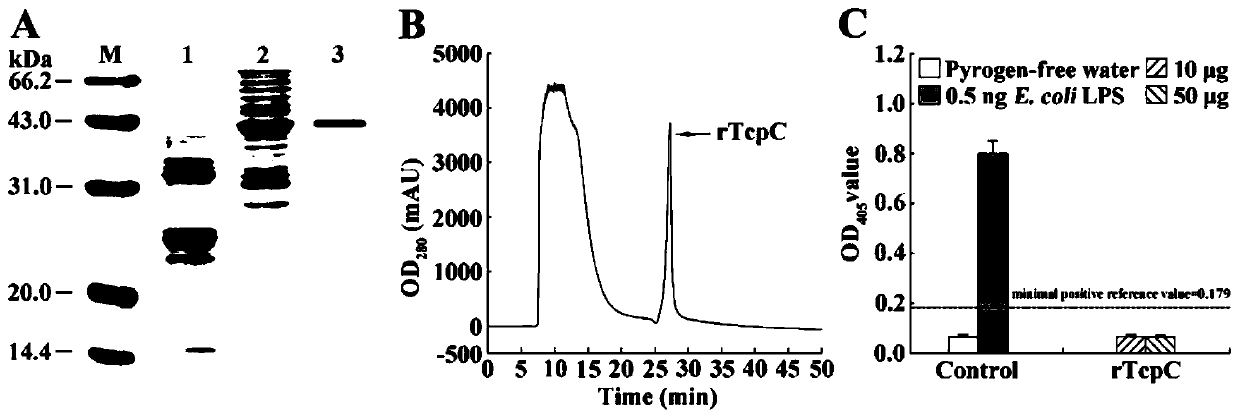
[0032] Example 1: Nucleotide (SEQ ID No. 1) and amino acid sequence (SEQ ID No. 2) of GenBank (GQ903014.1) TcpC.
Embodiment 2
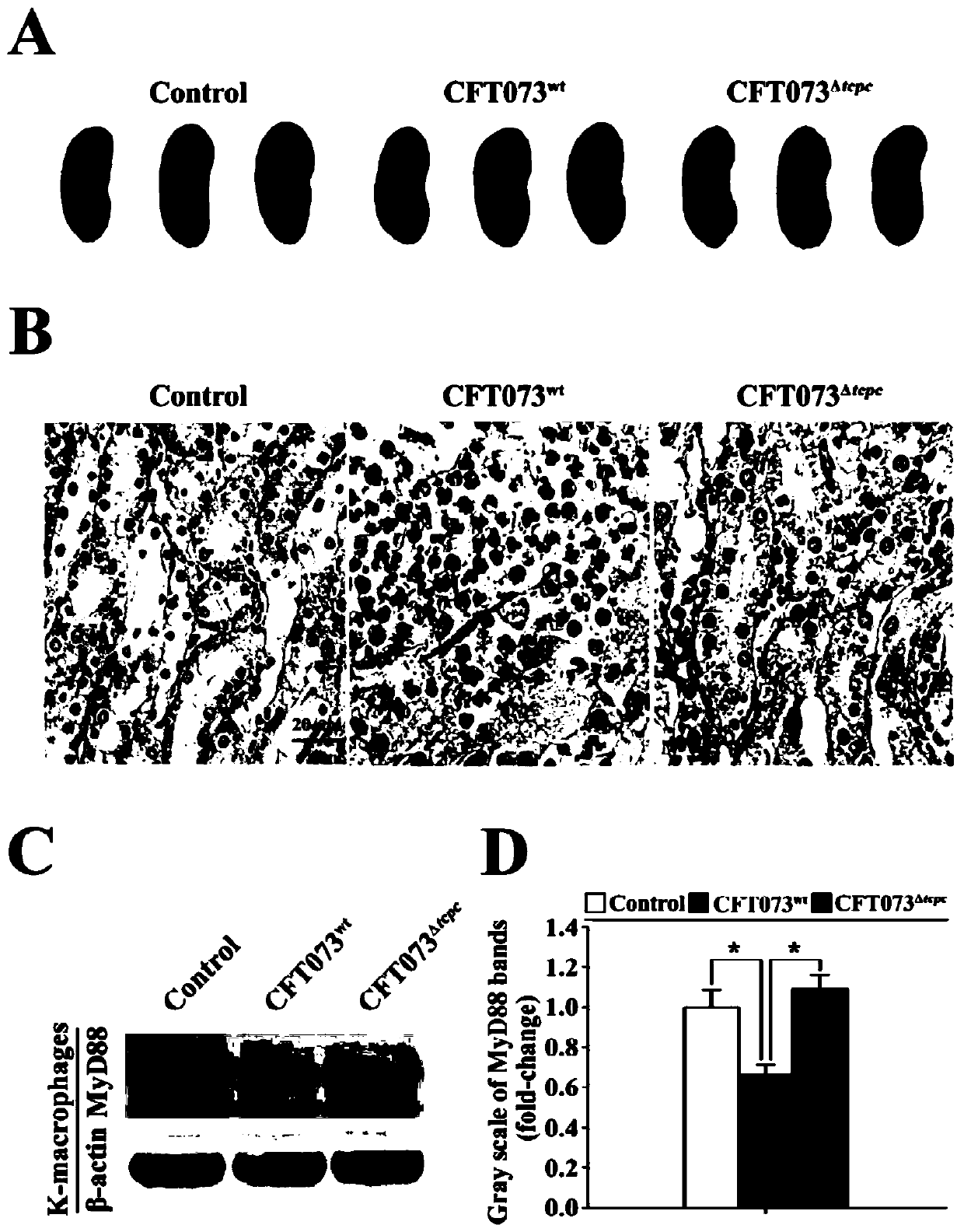
[0033] Example 2: Construction and validation of pyelonephritis model mice and detection of MyD88 protein level in renal macrophages.
[0034] We have secreted TcpC CFT073 wild strain (CFT073 wt ) on the basis of gene homologous recombination to construct the CFT073 mutant strain (CFT073 Δtcpc ).
[0035] 1. Construction and verification of pyelonephritis model mice
[0036] 1) The CFT073 wt 、CFT073 Δtcpc Cultivate in LB liquid medium to the logarithmic growth phase, centrifuge at 5500rpm / min at 4°C for 30min, discard the supernatant, wash the bacteria three times with LB medium and resuspend to 1×10 10 CFU / mL.
[0037] 2) 24 female C57BL / 6 mice of SPF grade, 6-8 weeks old were selected and divided into blank control group, CFT073 wt group with CFT073 Δtcpc groups, with 8 mice in each group.
[0038] 3) Anesthetize the mouse with Avertin (40mg 2,2,2-tribromoethanol dissolved in 1mL tert-butanol) (intraperitoneal 0.01mL / g), and instill 0.1mL from the urethra to the blad...
Embodiment 3
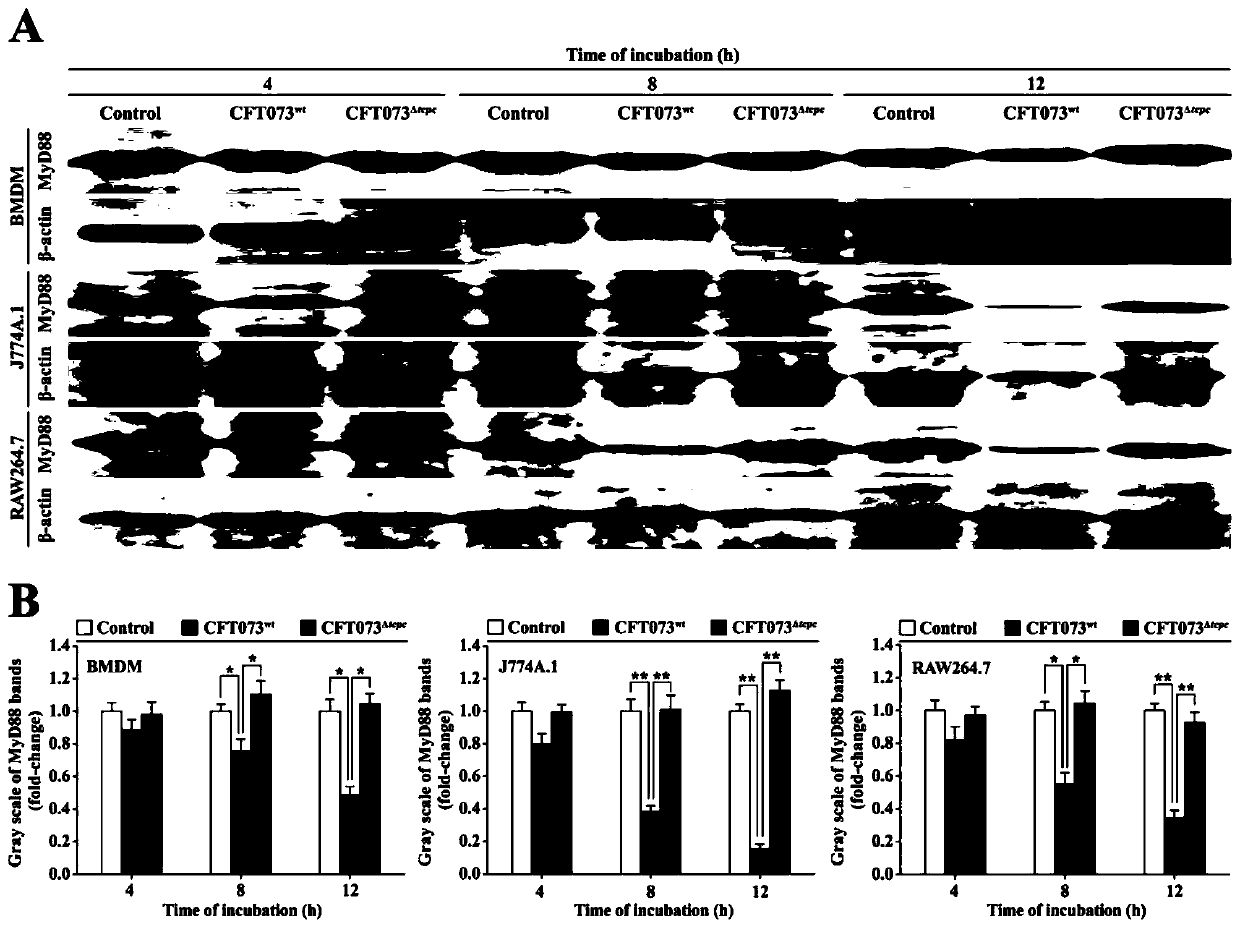
[0078] Example 3: CFT073 wt Inhibition of MyD88 protein levels in macrophages.
[0079] 1. Preparation of mouse bone marrow macrophages (BMDM): SPF grade C57BL / 6 mouse bone marrow was aseptically taken, and bone marrow cell suspension was prepared with DMEM medium. Centrifuge with the density gradient of lymphocyte separation medium, absorb the mononuclear cell layer, wash with PBS and suspend in DMEM medium containing M-CSF (10ng / mL), adjust the cell concentration to 1×10 7 / mL, inoculated into 6-well plates. 37°C, 5% CO 2 After 3 days of culture, the adherent cells were supplemented with fresh medium, and the adherent cells were collected on the 7th day. BMDM were obtained by CD11b immunomagnetic bead sorting, and then CD11b was detected by flow cytometry + and F4 / 80 + Cell Purity.
[0080] 2. BMDM, macrophage cell line (J774A.1, RAW264.7) (5 × 10 5 ) respectively with CFT073 wt or CFT073 Δtcpc The co-cultivation was separated by Transwell (MOI=100) for 4, 8, and 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com