Cyclodextrin glucosyltransferase mutant T168A and preparation method thereof
A glucosyl and cyclodextrin technology used in the fields of genetic engineering and enzyme engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
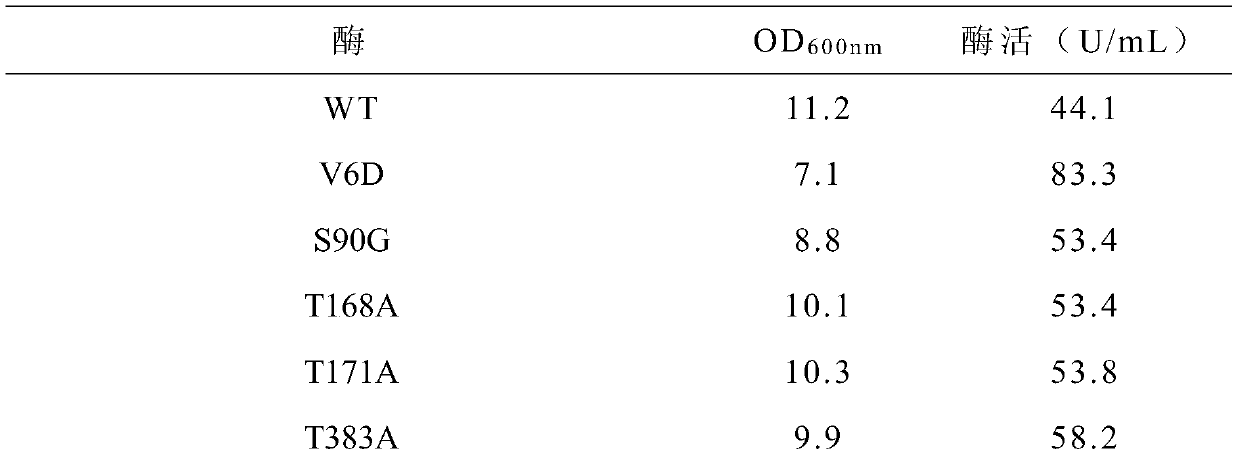
[0029] Example 1: Expression of wild-type cyclodextrin glucosyltransferase
[0030] Inoculate Cgt / pET20b(+) / BL21(DE3) (Yang Yulu, Wang Lei, Chen Sheng, etc.) from the glycerol tubes preserved in the laboratory in the early stage. The production process of β-cyclodextrin glucosyltransferase Condition optimization [J]. Biotechnology Bulletin, 2014, 8:175-181.) Grow in LB liquid medium (containing 100mg / L ampicillin) for 8 hours, and insert the seed solution into TB liquid fermentation medium according to 5% inoculum size (containing 100mg / L ampicillin). After Escherichia coli was cultured and fermented on a shaking table at 25°C for 48 hours, a certain volume of fermentation broth was centrifuged at 4°C and 12,000 rpm for 15 minutes, and the fermentation supernatant was taken, which was the crude enzyme liquid of the wild enzyme.
Embodiment 2
[0031] Example 2: Preparation and expression of cyclodextrin glucosyltransferase single mutant
[0032] (1) Single mutation preparation of cyclodextrin glucosyltransferase
[0033] According to the gene sequence of Bacillus circulans cyclodextrin glucosyltransferase, primers were designed and synthesized to introduce a single mutation, the cyclodextrin glucosyltransferase gene Cgt was subjected to site-directed mutation, and the cyclodextrin glucosyltransferase mutants were confirmed by sequencing Whether the coding gene is correct; the vector carrying the mutant gene is introduced into Escherichia coli for expression, and a single mutant cyclodextrin glucosyltransferase is obtained.
[0034] PCR amplification of the gene encoding the site-directed mutant: using the rapid PCR technique, the expression vector Cgt / pET-20b(+) carrying the gene encoding wild-type cyclodextrin glucosyltransferase was used as a template.
[0035] The site-directed mutagenesis primers for introducing ...
Embodiment 3
[0070] Example 3: Preparation and expression of cyclodextrin glucosyltransferase six mutants
[0071] (1) Preparation of six mutations of cyclodextrin glucosyltransferase
[0072] The plasmid carrying the gene encoding the mutant V6D constructed in Example 2 was used as the template for the six mutations, and the primers for site-directed mutation of S90G, T168A, T383A, and G608A designed according to Example 2 were used for rapid PCR to carry the encoding mutant. The plasmid of the V6D gene was subjected to site-directed mutation to obtain five mutants of cyclodextrin glucosyltransferase V6D / S90G / T168A / T383A / G608A. Afterwards, using the plasmid of the V6D / S90G / T168A / T383A / G608A five mutants as a template, new primers were designed to introduce the T171A mutation to construct the cyclodextrin glucosyltransferase V6D / S90G / T168A / T171A / T383A / G608A six mutants body.
[0073] The site-directed mutagenesis primers for introducing the T171A mutation were changed to:
[0074] The f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com