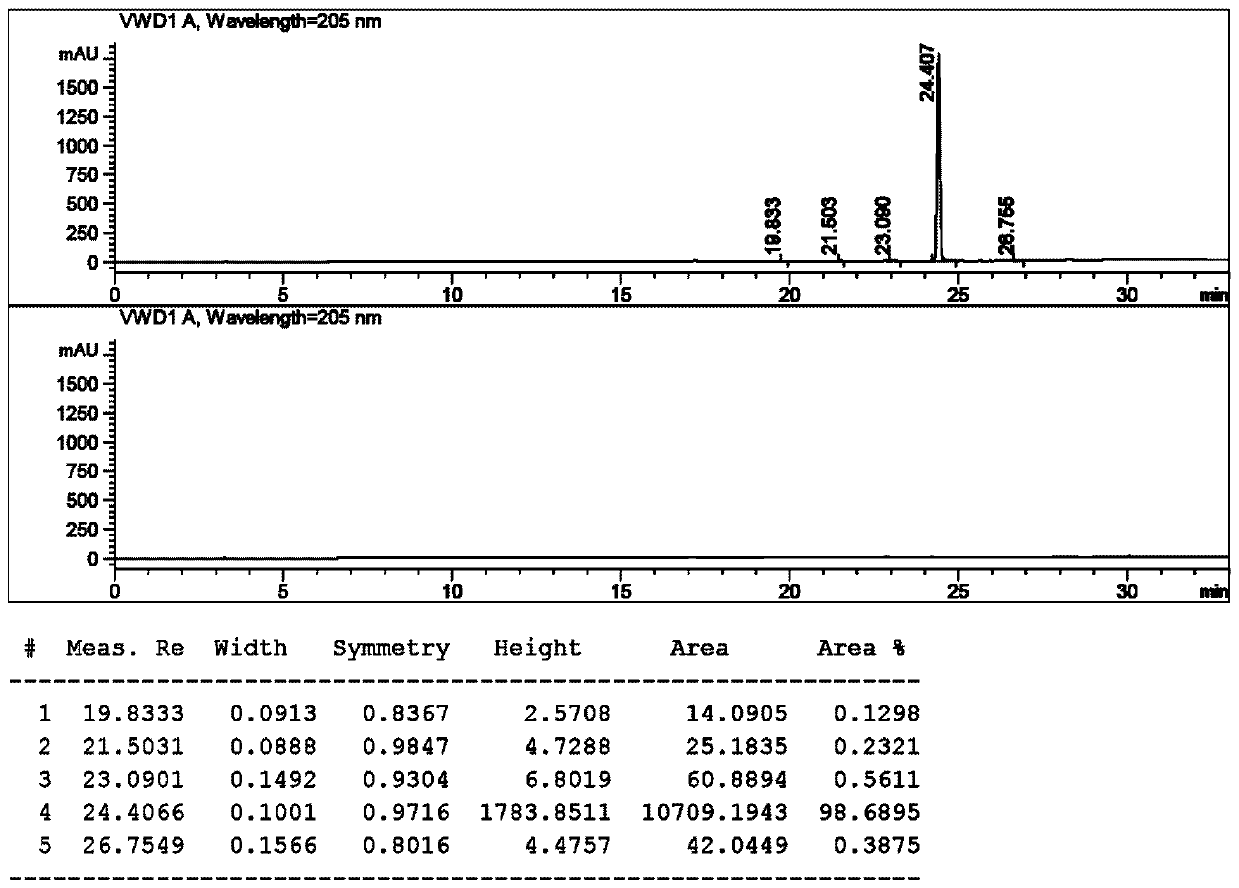
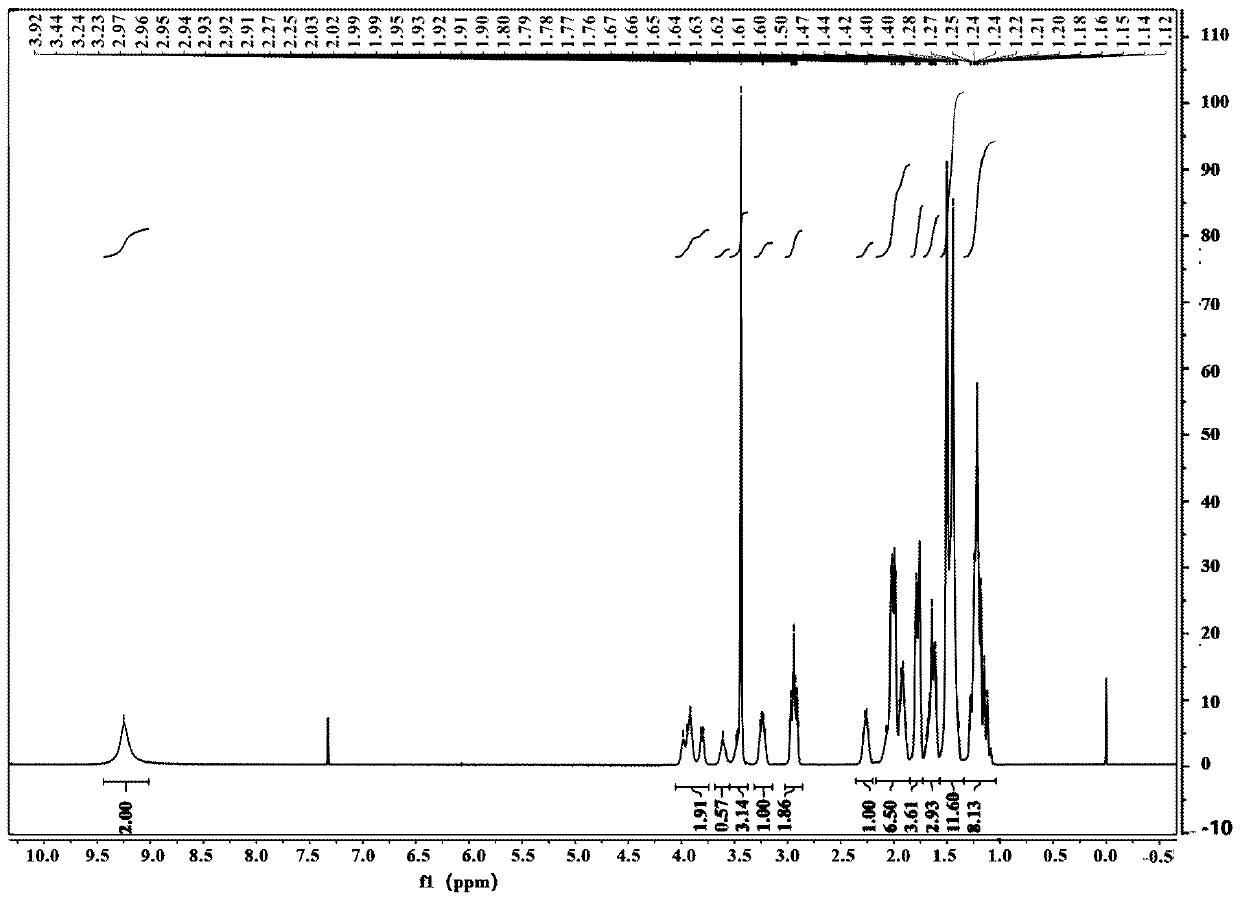
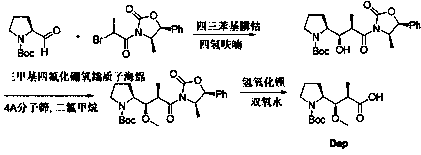
The synthetic method of n-boc-dolaproine and boc-dap DCHA
A synthesis method and reaction technology, which is applied in the field of medicine and chemical industry, can solve the problems of low selectivity, unfriendly environment, three wastes, etc., and achieve the effect of simple operation of the synthesis process, easy purification, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of compound 3:
[0052] X=I, R=cyclopropyl, solvent is dioxane
[0053] Add 20 volumes of dioxane and 3.0 equivalents of zinc powder to the glass bottle, then replace the air until the oxygen content is ≤1.0%, then add 0.5 equivalents of trimethylchlorosilane dropwise, and raise the temperature of the system to 50~60°C. Stir the reaction for 2-3 hours. Then the system was cooled to 30~35°C, and 1.0 equivalent of N-Boc-L-prolinaldehyde dioxane solution was added dropwise to the reaction system, and stirred for 25 minutes. Then slowly add 0.8 equivalent of compound 2 in dioxane solution dropwise to the system, first drop about 20% compound 2 solution, after stirring for 0.5~1h, continue to drop the remaining compound 2 solution. The temperature was controlled at 30° C., and the reaction was continued for 4 hours. HPLC tracking, until the remaining compound 2 is less than 5%. The reaction system was quenched with saturated ammonium chloride, and the organic p...
Embodiment 2
[0055] Synthesis of compound 3:
[0056] X=Cl, R=cyclohexane, the solvent is 2-methyltetrahydrofuran
[0057] Add 20 volumes of 2-methyltetrahydrofuran and 3.0 equivalents of zinc powder to the glass bottle, then replace the air until the oxygen content is ≤1.0%, and then add 0.5 equivalents of trimethylchlorosilane dropwise; raise the temperature of the system to 50~60°C , stirring and reacting for 2 to 3 hours. Then the system was cooled to 30~35°C, and 1.0 equivalent of N-Boc-L-prolinaldehyde solution in 2-methyltetrahydrofuran was added dropwise to the reaction system, and stirred for 25 minutes. Then slowly add 0.8 equivalent of compound 2 in 2-methyltetrahydrofuran solution dropwise to the system, first drop about 20% compound 2 solution, after stirring for 0.5~1h, continue to drop the remaining compound 2 solution. The temperature was controlled at 30° C., and the reaction was continued for 4 hours. HPLC tracking, until the remaining compound 2 is less than 5%. The ...
Embodiment 3
[0059] Synthesis of compound 3:
[0060] X=Br, R=cyclohexane, solvent is tetrahydrofuran. Among them, Zn: 1.0 equivalent, dropping temperature: 10~20°C.
[0061]Add 20 volumes of tetrahydrofuran and 1.0 equivalent of zinc powder to the glass bottle, then replace the air until the oxygen content is ≤1.0%, and then add 0.5 equivalent of trimethylchlorosilane dropwise; raise the temperature of the system to 40~50°C, and stir the reaction for 1 ~2 hours. Then the temperature of the system was lowered to 10~20°C, and 1.0 equivalent of N-Boc-L-prolinaldehyde tetrahydrofuran solution was added dropwise to the reaction system, and stirred for 20~25min. Then slowly add 0.8 equivalent of compound 2 in tetrahydrofuran solution dropwise to the system, first drop about 20% compound 2 solution, after stirring for 0.5~1h, continue to drop the remaining compound 2 solution. Control the temperature at 30°C and continue the reaction for 3 to 4 hours. HPLC tracking, until the remaining compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com