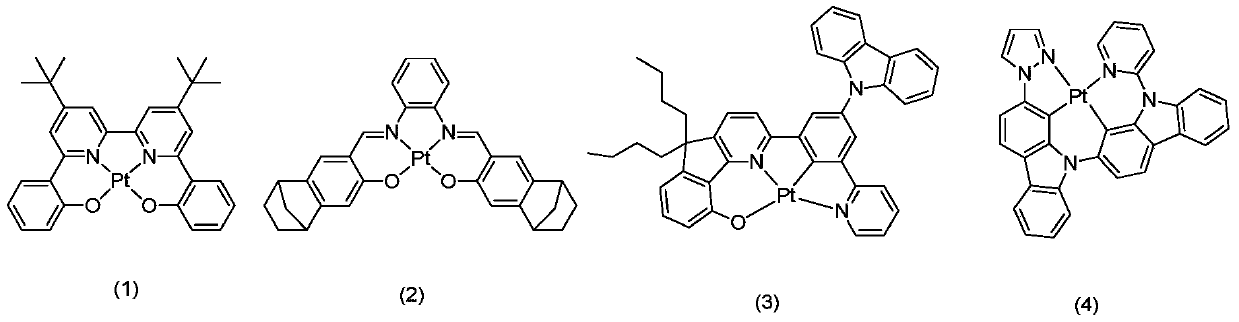
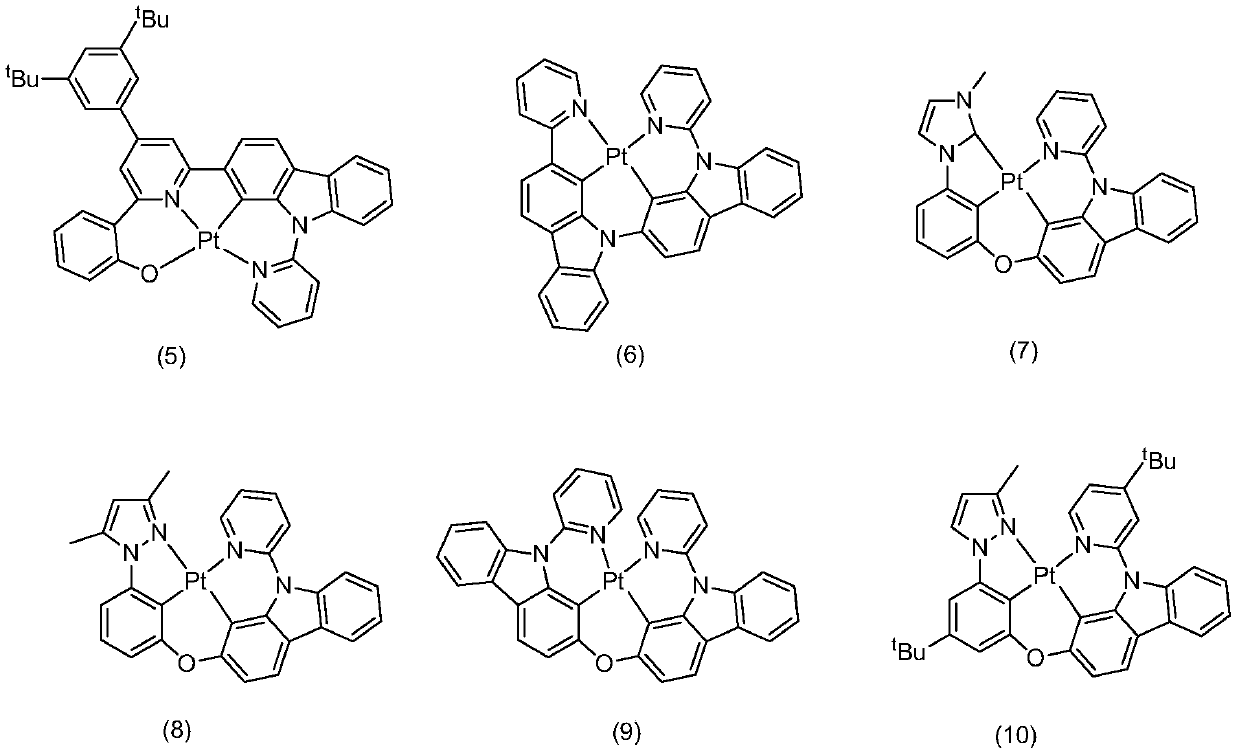
Preparation and application of N'N'C'N type tetradentate platinum (II) complex
A technology of N^N^C^N and complexes, applied in the field of phosphorescent doped materials, can solve the problems of easy aging, short service life, and restrictions on the large-scale application of OLED technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The preparation method of above-mentioned complex, comprises the steps:
[0055] As shown below, carbazole derivative S1 is subjected to bromination reaction to obtain substrate S2, S2 is reacted with biboronic acid pinacol ester to obtain corresponding pinacol ester derivative S3, and S3 is obtained by Suzuki reaction with pyridine derivative S6 S7, S7 and pyridine derivatives S8 were reacted by Suzuki to obtain S9, S9 and K 2 PtCl 4 The reaction results in the target product P. Among them, S6 is prepared by Stille reaction of S4 and S5.
[0056]
Embodiment 1
[0060]
[0061] synthetic route:
[0062]
[0063] Synthesis of Compound 2: Take 11.2g (40.0mmol) of Compound 1, dissolve it in 600mL of acetic acid, then drop into 16.0g (2.5eq., 100.0mmol) of liquid bromine, and react in a light-shielding manner. After stirring at room temperature for about 4 hours, remove the solvent by rotary evaporation, then add an appropriate amount of water and sodium bisulfite solution to wash, extract with ethyl acetate, collect the organic phase, dry it over anhydrous magnesium sulfate, add an appropriate amount of silica gel, remove the solvent by rotary evaporation, and use n-hexane / ethyl acetate system column chromatography to obtain 15.7 g of a white solid with a yield of 90% and a purity of 99.9%.
[0064] Synthesis of compound 6: take 14.7g (40.0mmol) compound 5, 34.0g compound 4 (3eq., 120.0mmol) and Pd (PPh 3 ) 4 924mg (0.02eq., 0.8mmol), was added to a three-necked flask, evacuated and replaced with nitrogen several times, then in...
Embodiment 2
[0069]
[0070] The synthesis route of P2 is basically the same as that of P1, and the synthesis of some compounds is shown below.
[0071]
[0072] Synthesis of compound 11: take 15.3g (40.0mmol) compound 10, 34.0g compound 4 (3eq., 120.0mmol) and Pd (PPh 3 ) 4 924mg (0.02eq., 0.8mmol), was added to a three-necked flask, evacuated and replaced with nitrogen several times, then injected with 200mL of toluene, and heated to 105°C. After reacting for 12 hours under the protection of nitrogen, cool to room temperature, quench the reaction with KF solution, add an appropriate amount of water and ethyl acetate to extract, collect the organic phase, dry over anhydrous magnesium sulfate and remove the solvent by rotary evaporation, use n-hexane / ethyl acetate System column chromatography obtained 8.4 g of white solid with a yield of 85% and a purity of 99.0%.
[0073] The synthesis of compound 12: get 10.3g (20.0mmol) compound 3, 5.0g (20.0mmol) compound 11, potassium carbona...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com