Microbial limit detection method of cross-linking or high molecular hyaluronic acid type substances
A microbial limit and high molecular weight technology, applied in the field of hyaluronic acid microbial detection, can solve the problems of unsuitable microbial reproduction, microbial proliferation, etc., to solve the problem of inability to accurately measure microbial limit, inhibit proliferation, and reduce the difficulty of formulation and coating. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
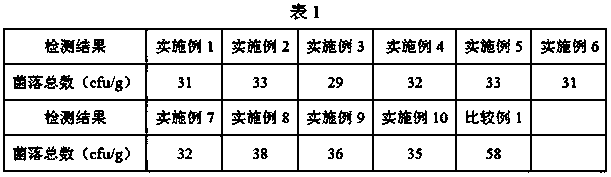
Examples
Embodiment 1
[0036] (1) Preparation of sterile hyaluronidase solution
[0037] will contain 100,000 IU of Bacillus ( Bacillus sp.) A50 CGMCC NO.5744 producing hyaluronidase, sterilized and filtered, added to 5 mM sterile phosphate buffer (pH5.0) containing appropriate amount of glass dispersed beads, to prepare sterile hyaluronidase containing Phosphate solution 90 mL.
[0038] (2) Preparation of samples to be tested
[0039] Weigh 10 g of high molecular weight sodium hyaluronate, the molecular weight of the high molecular weight sodium hyaluronate is 2800 kDa, and the dynamic viscosity of its 0.1 wt% aqueous solution is 280 mPa·s. Add 10 g of high molecular weight sodium hyaluronate to the above 90 mL of sterile phosphate solution containing hyaluronidase, oscillate and mix well to disperse and suspend, shake in a water bath at 15°C for 40 minutes, and the obtained enzyme The hydrolysis solution is uniform and transparent, with no obvious viscosity visible to the naked eye, then take ...
Embodiment 2
[0049] (1) Preparation of sterile hyaluronidase solution
[0050] will contain 150,000 IU of Bacillus ( Bacillus sp.) A50 CGMCC NO.5744 hyaluronidase producing hyaluronidase was sterilized and filtered, added to 50 mM sterile phosphate buffer (pH4.5) containing appropriate amount of glass dispersion beads to prepare sterile hyaluronidase containing Phosphate solution 90 mL.
[0051] (2) Preparation of samples to be tested
[0052] Weigh 10 g of cross-linked sodium hyaluronate, the dynamic viscosity of the cross-linked sodium hyaluronate 0.1wt% aqueous solution is 450 mPa·s. Add 10 g of cross-linked sodium hyaluronate to the above 90 mL of sterile phosphate solution containing hyaluronidase, shake and mix well to make it dispersed and suspended, shake in a water bath at 18°C for 60 minutes, and the obtained enzyme The hydrolysis solution is uniform and transparent, with no obvious viscosity visible to the naked eye, then take 10 mL of the enzymatic hydrolysis solution, ad...
Embodiment 3
[0055] (1) Preparation of sterile hyaluronidase solution
[0056] will contain 3,000,000 IU of Bacillus ( Bacillus sp.) A50 CGMCC NO.5744 producing hyaluronidase, sterilized and filtered, added to sterile phosphate buffer (pH4.8) containing appropriate amount of glass dispersion beads 100 mM, to prepare sterile phosphoric acid containing hyaluronidase Saline solution 90 mL.
[0057] (2) Preparation of samples to be tested
[0058] Weigh 10 g of cross-linked magnesium hyaluronate, the dynamic viscosity of the cross-linked magnesium hyaluronate 0.1wt% aqueous solution is 1500mPa·s. Add 10 g of cross-linked magnesium hyaluronate to the above-mentioned 90 mL of sterile phosphate solution containing hyaluronidase, shake and mix well to disperse and suspend, shake in a water bath at 10°C for 120 minutes, and the obtained enzyme The hydrolysis solution is uniform and transparent, with no obvious viscosity visible to the naked eye. Then take 10 mL of the enzymatic hydrolysis solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com