MOF-based composite material and preparation method and application thereof
A composite material and processing method technology, applied in the field of MOF-based composite materials and their preparation, can solve the problems of unsatisfactory visible light response, increase the concentration of active substances, etc., and achieve high visible light activity and stability, low cost, and good visible light catalytic activity. and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of MOF-based photocatalyst FePMo / MIL-53 (Fe), the steps are as follows:
[0036] (1) Disperse 0.32g ferric chloride and 0.33g terephthalic acid in 41mL N,N-dimethylformamide in sequence to obtain the MIL-53(Fe) precursor solution, which is transferred to the reaction kettle at 150 The solvothermal reaction was carried out in an oven at ℃ for 15 hours. After the reaction, it was centrifuged with methanol, dried in a vacuum oven at 80° C. for 10 h, and ground with an agate mortar to obtain MIL-53(Fe).
[0037] (2) Evenly disperse 0.034g of ferric nitrate in 20mL of methanol to obtain Fe 3+ Solution; 0.155g of phosphomolybdic acid was uniformly dispersed in 20mL of methanol to obtain a phosphomolybdic acid solution. Will Fe 3+ The solution was added dropwise into the phosphomolybdic acid solution to obtain the FePMo precursor solution.
[0038] (3) Disperse 0.2g MIL-53(Fe) evenly in the FePMo precursor solution, transfer it to the reaction kettle...
Embodiment 2
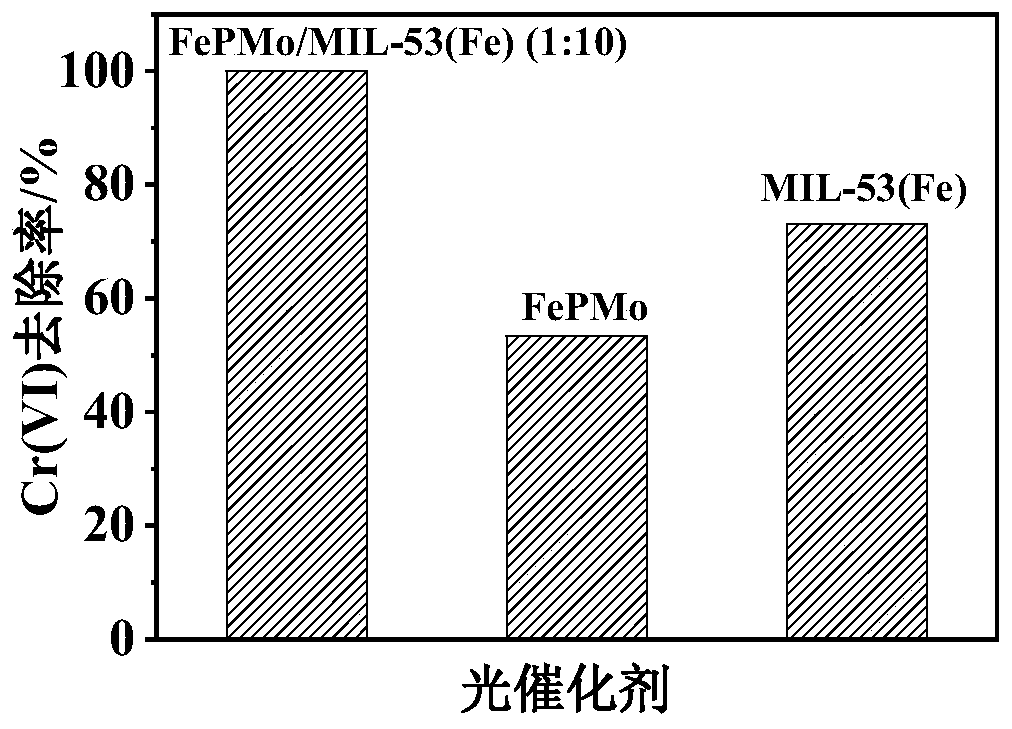
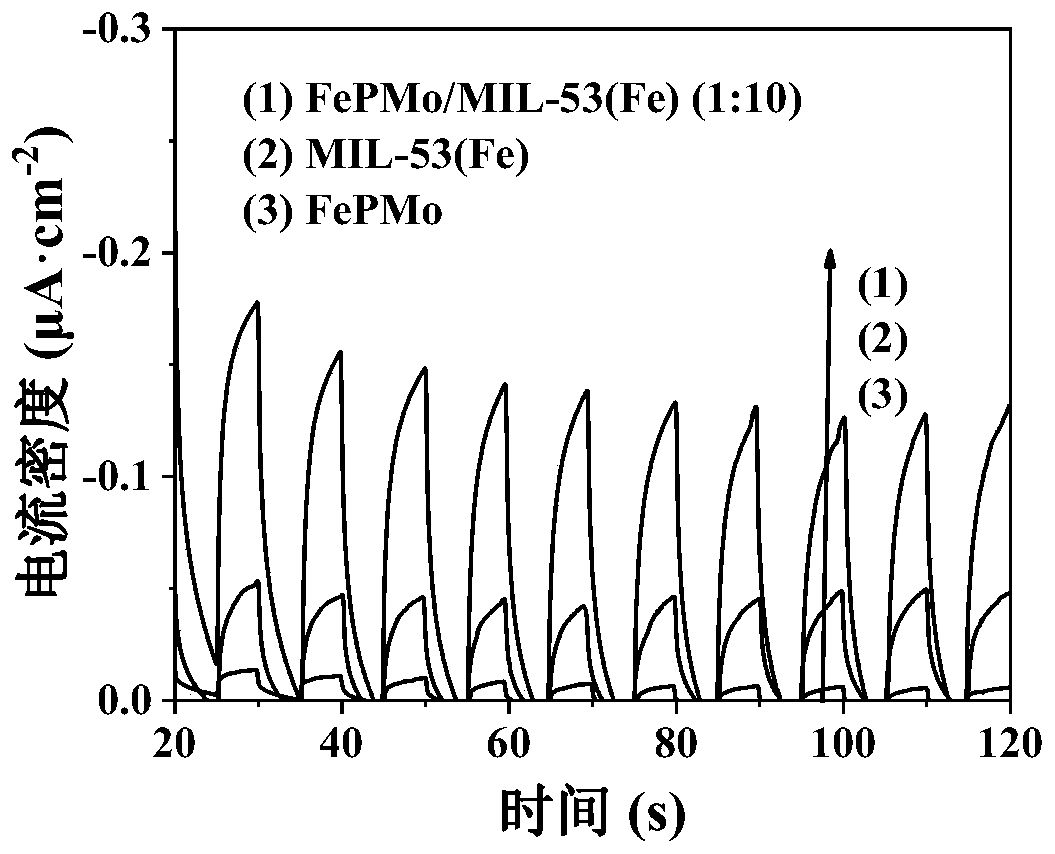
[0044] Take 0.05g of the catalyst FePMo, MIL-53(Fe) and FePMo / MIL-53(Fe) (1:10) prepared in Example 1 and dissolve in 0.5mL Nafin solution, ultrasonically disperse for 15min, and drop-coat it on the conductive glass , the size of the drop coating is 1cm×1cm, coating 2 times, each drop coating 20μL, and drying in a vacuum oven at 80°C for 1h to prepare FePMo, MIL-53(Fe) and FePMo / MIL-53(Fe) (1 :10) Electrodes.
[0045] The prepared three photocatalytic electrodes were respectively placed in 0.1mol / L Na 2 SO 4 and Na 2 SO 3 In the solution, under the 3-electrode system of the electrochemical workstation, the conductive glass coated with photocatalyst is used as the working electrode, the platinum sheet is used as the counter electrode, the Ag / AgCl electrode is used as the reference electrode, and the xenon lamp is used as the light source. Under visible light, light and dark alternate at a certain interval , to obtain the time-current curves of different photocatalysts, the ...
Embodiment 3
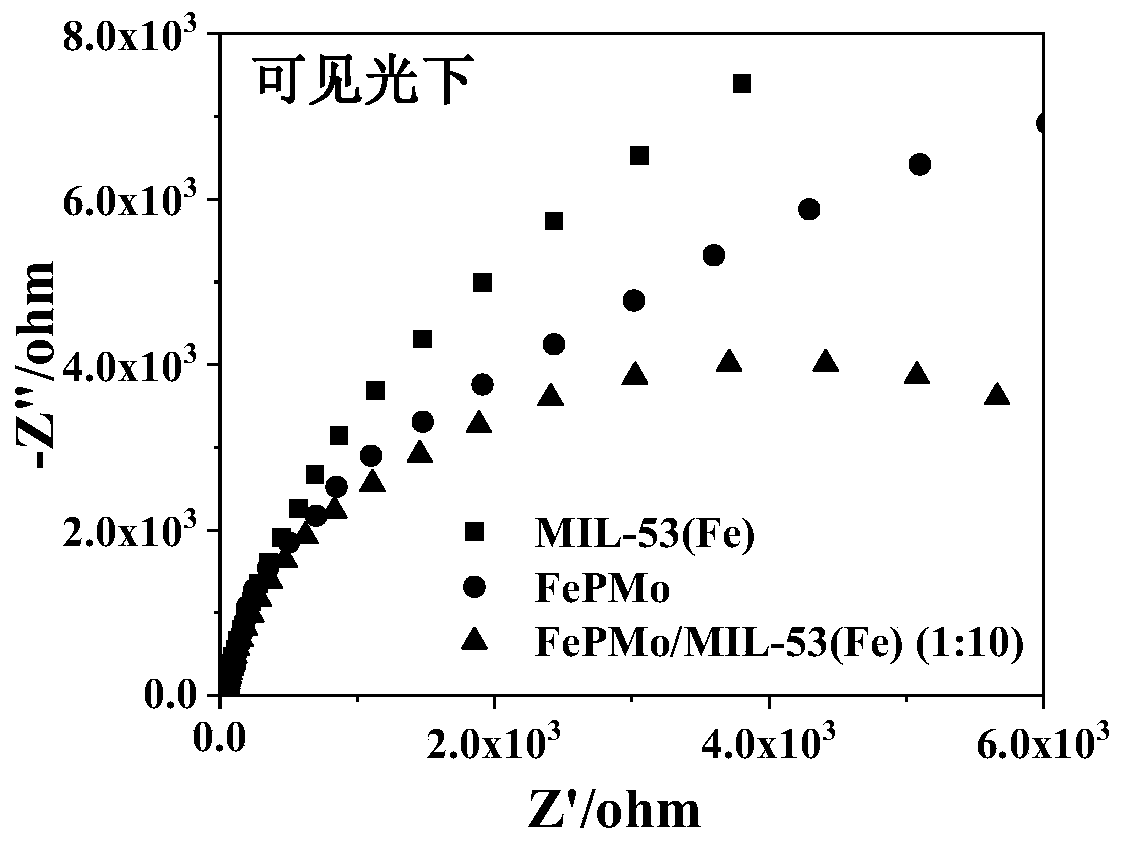
[0048] The electrode prepared in Example 2 was placed in 0.5M Na 2 SO 4 In the solution, electrochemical impedance spectroscopy was performed under visible light to obtain image 3 . From image 3 It can be seen that the arc radius of the electrochemical impedance spectrum of FePMo / MIL-53(Fe)(1:10) under visible light is the smallest, indicating that the introduction of FePMo into the MIL-53(Fe) framework makes the photogenerated carriers generated on the surface of the catalyst The transfer rate increases, the charge transfer resistance decreases, and the separation rate of photogenerated electrons and holes on the interface becomes faster.
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com