Use of NSC228155 in preparation of medicine for preventing and treating acute kidney injury
1. NSC228155, acute kidney injury technology, applied in the field of medicine, can solve the problems of lack of pharmacological activity of acute kidney injury, NSC228155 has not been found, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
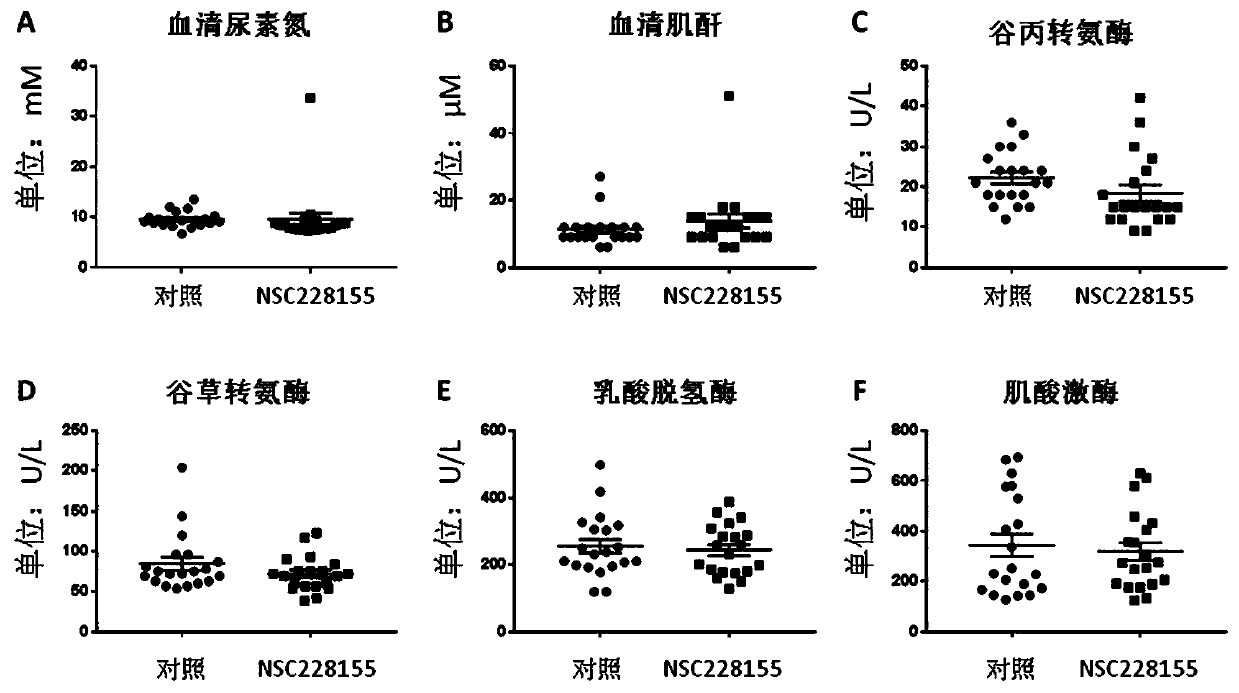
[0045] Example 1 The therapeutic dose of NSC228155 has no toxic side effects on mice
[0046] 1 Experimental materials and methods
[0047] 1) Administration, feeding and sampling of mice
[0048] The C57BL / 6 species male mice used in the present invention (7 weeks old at the time of purchase, body weight 20-24g) were purchased from the Experimental Animal Center of Nanjing Medical University, raised in an SPF level barrier environment of the Experimental Animal Center of Nanjing Medical University, and the animals were free to eat , maintaining a circadian rhythm of 12 hours of light and 12 hours of darkness. Laboratory temperature: 20-25°C, humidity 50±5%. After 1 week of adaptive feeding, the mice were randomly divided into control group (n=20), NSC228155 group (n=20), cisplatin group (n=20), cisplatin+NSC228155 group (n=20), and the former Two groups can be used to evaluate the safety of NSC228155 on mice under this test system.
[0049] The present invention uses NSC2...
Embodiment 2
[0054] Example 2NSC228155 improves renal function and renal pathological damage in mouse cisplatin model
[0055] 1 Experimental materials
[0056] The C57BL / 6 species mouse and NSC228155 (purity ≥ 99%) used in the present invention are from the same source as in Example 1.
[0057] 2 Experimental methods
[0058] 2.1 Animal administration, modeling and sampling
[0059] The present invention uses NSC228155 (purity≥99%) purchased from Selleck Company. Dilute to 0.25 mg / ml with vehicle (5% DMSO, 35% PEG-300, 65% sterile saline) before administration. The dosage of mice in NSC228155 group and cisplatin+NSC228155 group was 2.5mg / kg, administered by intraperitoneal injection, and the administration volume was 0.1ml per 10g of body weight; mice in the control group cisplatin group were given the same volume of the above vehicle by intraperitoneal injection , administered once a day for a total of 5 days. On the day of the third administration, the cisplatin group and the cispl...
Embodiment 3
[0075] Example 3NSC228155 reduces cisplatin-induced apoptosis in renal cortex tissue
[0076] 1. Experimental materials and methods
[0077] The source and use of mice and NSC228155 were as described in Example 2. The establishment method of the mouse cisplatin model and the tissue samples were the same as those in Example 2.
[0078] 1) TUNEL staining
[0079] Vazyme's TUNEL staining kit was used to dewax and hydrate the paraffin-embedded tissue sections, and the fragmented DNA in the tissue samples was fluorescently labeled according to the instructions, and the images were read and photographed using a laser confocal microscope.
[0080] 2) Western blot and RT-PCR
[0081] 3) Statistical analysis
[0082] Histograms represent data using mean ± SEM. Analysis of variance (ANOVA) was used for comparison among multiple groups. P<0.05 was regarded as statistically significant.
[0083] 2. Experimental results
[0084] Since cisplatin has a direct killing effect on renal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com