Application of NSC228155 in preparation of drug for preventing and treating chronic renal fibrosis
A 1. NSC228155, the technology of renal fibrosis, applied in the field of medicine, can solve the problem of lack of effective means for chronic kidney disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 The therapeutic dose of NSC228155 has no toxic side effects on mice and cells
[0046] 1 Experimental materials and methods
[0047] 1) Administration, feeding and sampling of mice
[0048] The C57BL / 6 species male mice used in the present invention (7 weeks old at the time of purchase, body weight 20-24g) were purchased from the Experimental Animal Center of Nanjing Medical University, raised in an SPF level barrier environment of the Experimental Animal Center of Nanjing Medical University, and the animals were free to eat , maintaining a circadian rhythm of 12 hours of light and 12 hours of darkness. Laboratory temperature: 20-25°C, humidity 50±5%. After one week of adaptive feeding, the mice were randomly divided into control group (n=10) and NSC228155 group (n=10).
[0049] The present invention uses NSC228155 (purity≥99%) purchased from Selleck Company. Dilute to 0.25 mg / ml with vehicle (5% DMSO, 35% PEG-300, 65% sterile saline) before administration...
Embodiment 2
[0058] Example 2 NSC228155 improves renal fibrosis in the UUO model
[0059] 1 Experimental materials
[0060] The C57BL / 6 species mouse and NSC228155 (purity ≥ 99%) used in the present invention are from the same source as in Example 1.
[0061] 2 Experimental methods
[0062] 2.1 Animal administration, modeling and sampling
[0063] Twenty male C57BL / 6 mice (7 weeks old at the time of purchase, weighing 20-24 g) were raised in an SPF-grade barrier environment in the Experimental Animal Center of Nanjing Medical University. The animals ate freely and maintained a circadian rhythm of 12 hours of light and 12 hours of darkness. Laboratory temperature: 20-25°C, humidity 50±5%. After one week of adaptive feeding, the mice were randomly divided into control group (n=10) and NSC228155 group (n=10). After grouping, the mice in the control group and the mice in the NSC228155 group were injected intraperitoneally with vehicle or NSC228155 once a day, for a total of 8 injections. ...
Embodiment 3
[0074] Example 3 NSC228155 reduces the expression level of fibrosis indicators in kidney tissue
[0075] 1. Experimental materials and methods
[0076] The source and use of mice and NSC228155 were as described in Example 2. The establishment method and tissue collection of the mouse UUO model are the same as in Example 2.
[0077] 1) RT-PCR
[0078] After RNAiso reagent from Takara company was used to extract the RNA in the sample according to the instructions, the RNA was reverse-transcribed into cDNA using the reverse transcription kit from Vazyme company, and SYBR green PCR mix combined with corresponding primers was used for RT-PCR detection.
[0079] 2) Statistical analysis
[0080] Histograms represent data using mean ± SEM. Analysis of variance (ANOVA) was used for comparison between multiple groups, and T test was used for data comparison between two groups. P<0.05 was regarded as statistically significant.
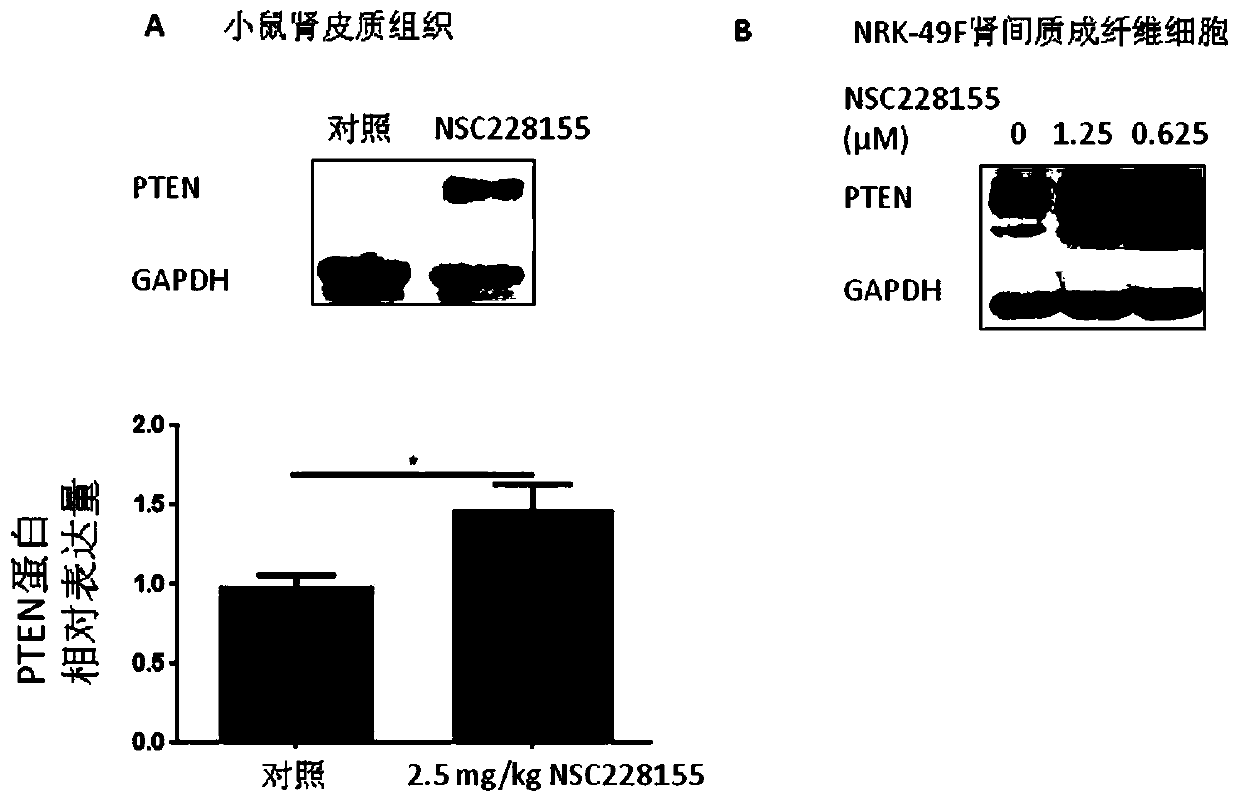
[0081] 2. Experimental results
[0082] In order to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com