A marine-sourced collagen-expanding protease vp9 and its coding gene and application
A technology that encodes genes and collagen, applied in applications, genetic engineering, plant genetic improvement, etc., can solve problems such as insufficient fiber unraveling and damage to tanning materials, and achieve good thermal stability, strong salt tolerance, and great application potential Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
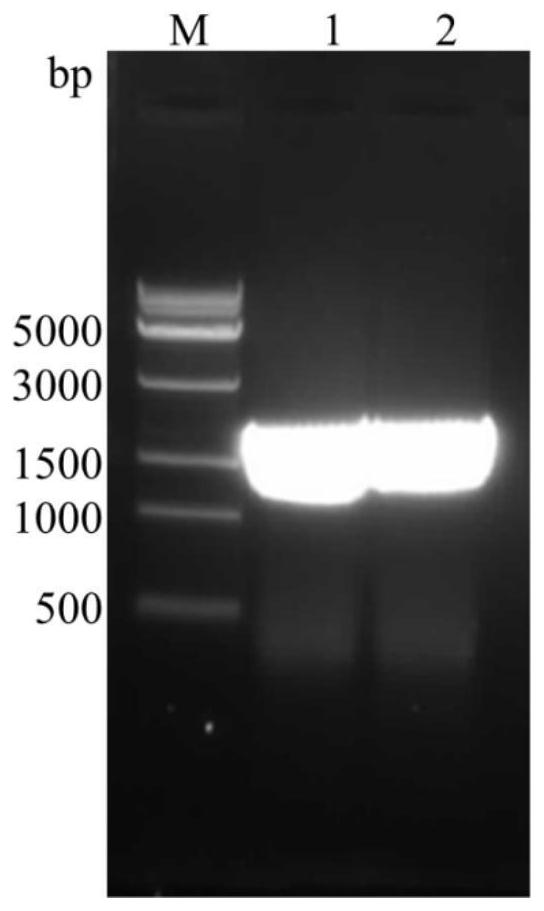
[0041] Example 1: Acquisition of Collagen Expansion Protease VP9 Encoding Gene and Construction of Its Expression Strain
[0042] 1. Genomic DNA extraction and whole genome sequencing of Vibrio pomeroyi strain 12613.
[0043] Genomic DNA was extracted from Vibrio pomeroyi strain 12613 according to the instructions of Genome Extraction Kit from Biotec (conventional extraction steps). Whole-genome sequencing was completed by Shanghai Meiji Biotechnology Co., Ltd.
[0044] 2. Purification of extracellular protease secreted by Vibrio pomeroyi strain 12613.
[0045] Vibrio pomeroyi strain 12613 was inoculated into the fermentation medium and cultured at 15°C for 60h. The fermentation broth was centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was dialyzed overnight with 50 mM Tris-HCl buffer (pH 8.0) and then centrifuged at 10000 rpm for 15 min at 4°C. The supernatant was passed through the DEAE-Sepharose Fast Flow chromatography column at a speed of 2.5min / mL and th...
Embodiment 2
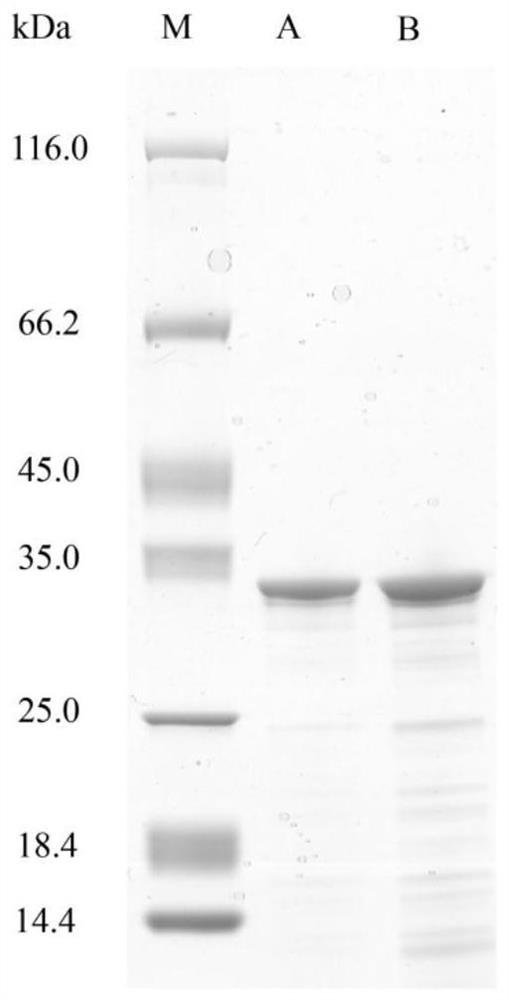
[0076] Example 2: Induced Expression and Purification of Collagen Expansion Protease VP9 Heterologous Expression Gene VP9 in Escherichia coli
[0077] 1. Fermentation of recombinant strains
[0078] (1) Cultivation of seeds
[0079] Pick the strain transferred to the recombinant pET-22b-VP9 expression vector on the plate to 100mL LB liquid medium with a final concentration of 100μg / mL ampicillin, and culture overnight at 37°C and 180rpm;
[0080] (2) Inoculate according to the inoculum amount of 1% by volume, and transfer the cultured seed liquid to a shake flask with a liquid volume of 1 L. Cultivate at 37°C and 180rpm until the absorbance of the bacterial solution is 0.8 at a wavelength of 600nm, then change the culture condition to 15°C and 100rpm, continue to cultivate for 30min, then add IPTG (isopropylthiogalactopyranoside) to a final concentration of 0.35mM , continue to cultivate for 16 hours;
[0081] 2. Purification of collagen expansion protease VP9
[0082] (1)...
Embodiment 3
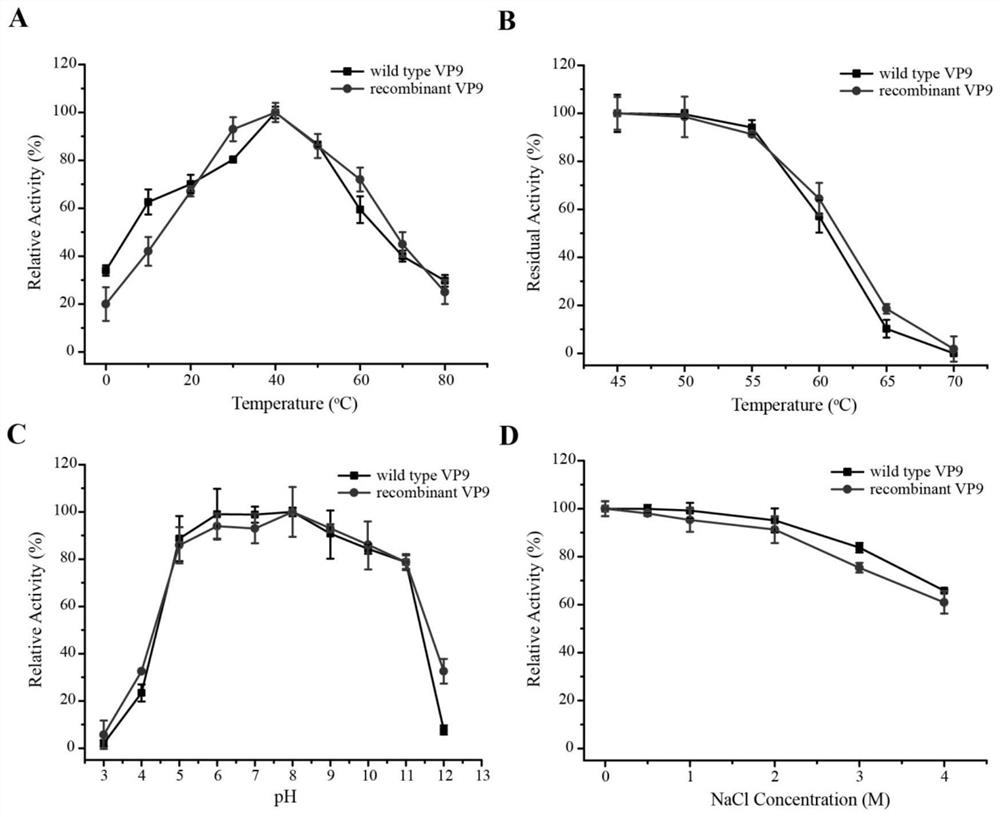
[0086] Embodiment 3: Derived from the property determination of the collagen expansion protease VP9 of wild-type strain and recombinant strain
[0087] 1. Optimum enzyme activity temperature analysis
[0088] The gelatinase activities of the two were measured at 0, 10, 20, 30, 40, 50, 60, 70, and 80°C to determine the optimum reaction temperature of the enzyme.
[0089] The specific method for measuring enzyme activity is as follows: prepare gelatin with a concentration of 2%, take 100 μL of substrate and add 100 μL of diluted enzyme solution, and react at 40° C. for 10 minutes. After the reaction was completed, 200 μL of 1.25 M pre-cooled TCA solution was added to terminate the reaction. Centrifuge at 4°C, 13,000 rpm for 10 minutes, take 20 μL of supernatant, add 100 μL of ninhydrin-sodium citrate mixture, mix well, boil in water bath for 20 minutes, take it out and add 500 μL of 50% n-propanol solution after cooling, mix well and measure at 600 nm color. The control group...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com