A kind of preparation method of imidacloprid
A technology of imidacloprid and chloromethylpyridinone, which is applied in the field of imidacloprid, can solve the problems of low yield of active ingredients and increased water content, and achieve the effect of reducing water content and increasing the content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a preparation method of imidacloprid, comprising the following steps:
[0027] Mixing 2-chloro-5-chloromethylpyridine butanone solution, butanone, phase transfer catalyst, imidazolidine and acid binding agent to obtain a mixed solution;
[0028] The mixed solution is subjected to a substitution reaction to obtain an initial reaction solution of imidacloprid;
[0029] The initial reaction solution of imidacloprid is sequentially desolvated and washed, and the organic phase is taken to obtain a purified reaction solution of imidacloprid, and the washing agent used in the washing includes water and butanone;
[0030] The imidacloprid purification reaction solution is crystallized to obtain the imidacloprid;
[0031] The acid binding agent includes one or more of trimethylamine, triethylamine and tripropylamine.
[0032] In the present invention, unless otherwise specified, all raw material components are commercially available products well known t...
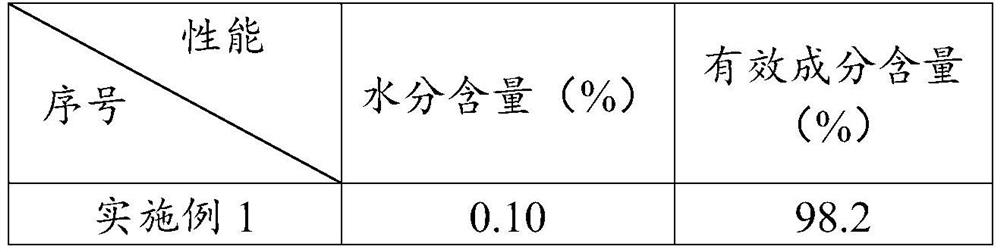
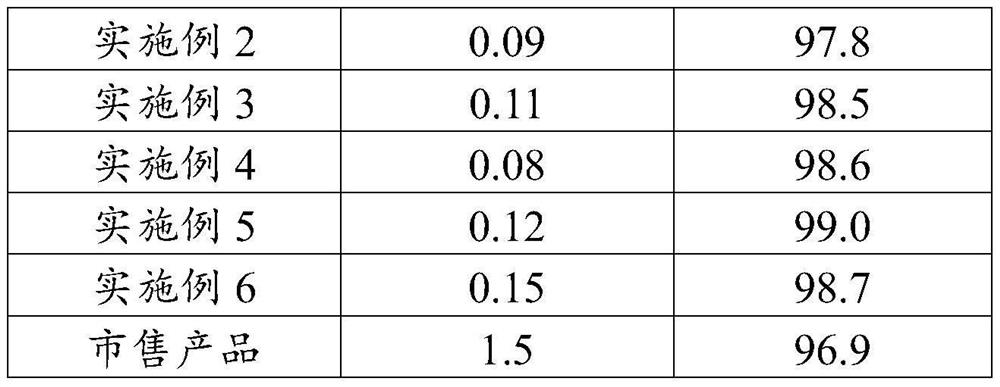
Embodiment 1
[0057] Mix 100g butanone and 32.4g 2-chloro-5-chloromethylpyridine in a dropping funnel to prepare 132.4g 2-chloro-5-chloromethylpyridine butanone solution, in which 2-chloro-5-chloromethylpyridine The mass concentration of pyridine is 24.4%;
[0058] In the reaction flask, 200g of butanone, 0.5g of tetra-tert-butylammonium bromide, 31g of imidazolidine and 35.2g of trimethylamine were premixed at 65°C with stirring at a rate of 300r / min to obtain a premixed solution;
[0059] 132.4g of 2-chloro-5-chloromethylpyridine butanone solution was added dropwise to the premixed solution at a rate of 25g / h. During the dropwise addition, the pH value of the system was measured every 30min. , keep the pH value of the system at 8, and after the dropwise addition is completed, a mixed solution is obtained;
[0060] The mixed solution is subjected to a substitution reaction at 65° C. and a stirring rate of 300 r / min for 4 h to obtain an initial reaction solution of imidacloprid;
[0061] ...
Embodiment 2
[0066] Mix 100g butanone and 32.4g 2-chloro-5-chloromethylpyridine in a dropping funnel to prepare 132.4g 2-chloro-5-chloromethylpyridine butanone solution, in which 2-chloro-5-chloromethylpyridine The mass concentration of pyridine is 24.4%;
[0067] In the reaction flask, premix 220g butanone, 0.45g tetra-tert-butylammonium bromide, 30.5g imidazolidine and 20.2g triethylamine at 65°C with stirring at a rate of 300r / min to obtain a premixed solution ;
[0068] 132.4g of 2-chloro-5-chloromethylpyridine butanone solution was added dropwise to the premixed solution at a rate of 25g / h. During the dropwise addition, the pH value of the system was measured every 30min. Amine, keep the pH value of the system at 8, after the dropwise addition is completed, a mixed solution is obtained;
[0069] The mixed solution is subjected to a substitution reaction at 65° C. and a stirring rate of 300 r / min for 4 h to obtain an initial reaction solution of imidacloprid;
[0070] The initial re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com