A kind of synthetic method of 2,4-dichloro-5-methoxypyrimidine
A technology of methoxypyrimidine and a synthesis method, applied in the field of fine chemical industry, can solve the problems of no economical efficiency, unfavorable industrialization, complicated post-processing and the like, and achieves the effects of reasonable selection of process conditions, easy operation and control, and avoidance of hydrolysis side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
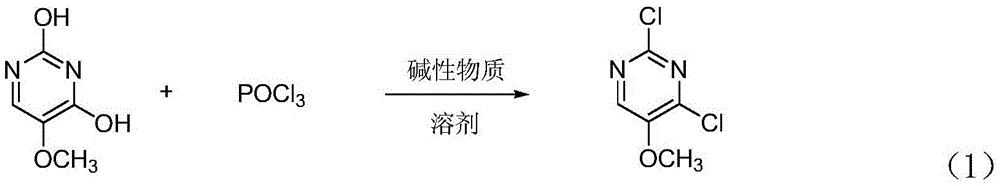
[0020] 2,4-dichloro-5-methoxypyrimidine synthetic method of the present invention, its reaction principle is as shown in formula (1), and the concrete steps of this synthetic method are as follows:
[0021]
[0022] (1) Add 2,4-dihydroxy-5-methoxypyrimidine, solvent and phosphorus oxychloride to the reaction flask after nitrogen replacement and mix uniformly, and carry out reflux reaction after adding alkaline substance under stirring condition; The ratio of 2,4-dihydroxy-5-methoxypyrimidine, solvent and phosphorus oxychloride is 1moL: (500~1000) mL: (2.0~2.5) mol; the basic substance and 2,4- The molar ratio of dihydroxy-5-methoxypyrimidine is (1.0~1.5): 1, and the basic substance is triethylamine, pyridine or N,N dimethylaniline; the solvent is toluene, xylene or trimethylbenzene; the reaction temperature of the reflux reaction is 100-160° C., and the reaction time is 2-6 hours.
[0023] (2) After cooling the system after the reflux reaction in the reaction bottle to 0-4...
Embodiment 1
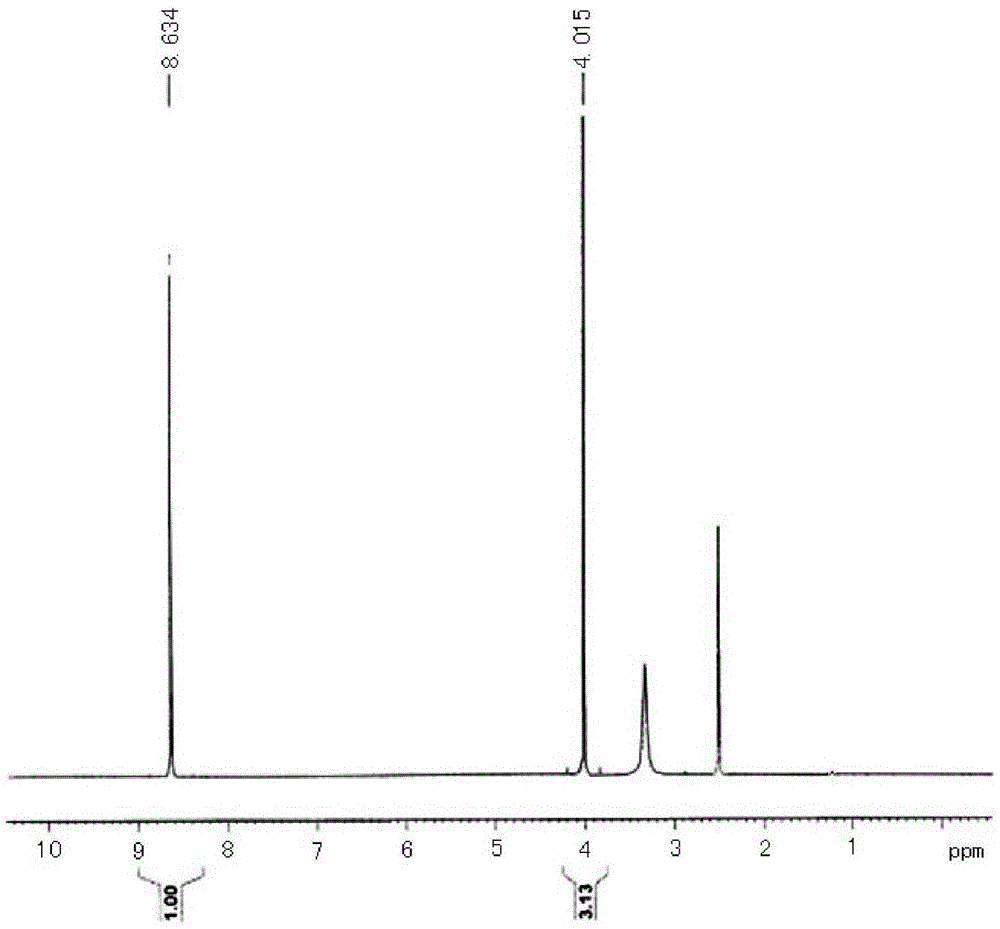
[0025] Put 14.3g (0.1mol, 99.5%, 1.0eq) of 2,4-dihydroxy-5-methoxypyrimidine, 100ml of toluene and 31.0g of phosphorus oxychloride (0.2mol, 99% , 2.0eq), start stirring, put in 12.0g of pyridine (0.15mol, 99%, 1.5eq), heat the system to 160°C for reflux reaction for 6h, the system becomes a brown-black solution, and LC detects that the reaction of raw materials is complete. The system was cooled to 10°C, 100ml of water was slowly added, stirred for 30min, separated into layers, the water layer was extracted once with 50ml of toluene, and the oil layers were combined for desolvation under reduced pressure to obtain 16.4g of the target product ( figure 1 ), the state is light yellow solid, the content (purity) is 98.6%, and the yield is 90.3%. 1H-NMR (400MHz, d6-DMSO) δ: 8.634 (1H, s), 4.015 (3H, s).
Embodiment 2
[0027] Put into 2,4-dihydroxy-5-methoxypyrimidine 14.3g (0.1mol, 99.5%, 1.0eq), xylene 80ml and phosphorus oxychloride 34.1g (0.22mol, 99 %, 2.2eq), stirring was started, and 12.3g (0.12mol, 99%, 1.2eq) of triethylamine was dropped in, and the system was heated to 160°C for reflux reaction for 4h, and the system became a brown-black solution, and the reaction of raw materials was detected by LC. Cool the system to 20°C, slowly add 80ml of water, stir for 30min, separate layers, extract the water layer once with 40ml of xylene, combine the oil layers and carry out desolvation under reduced pressure to obtain 17.0g of the target product in the form of a light yellow solid with a content of 98.5%. Yield 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com