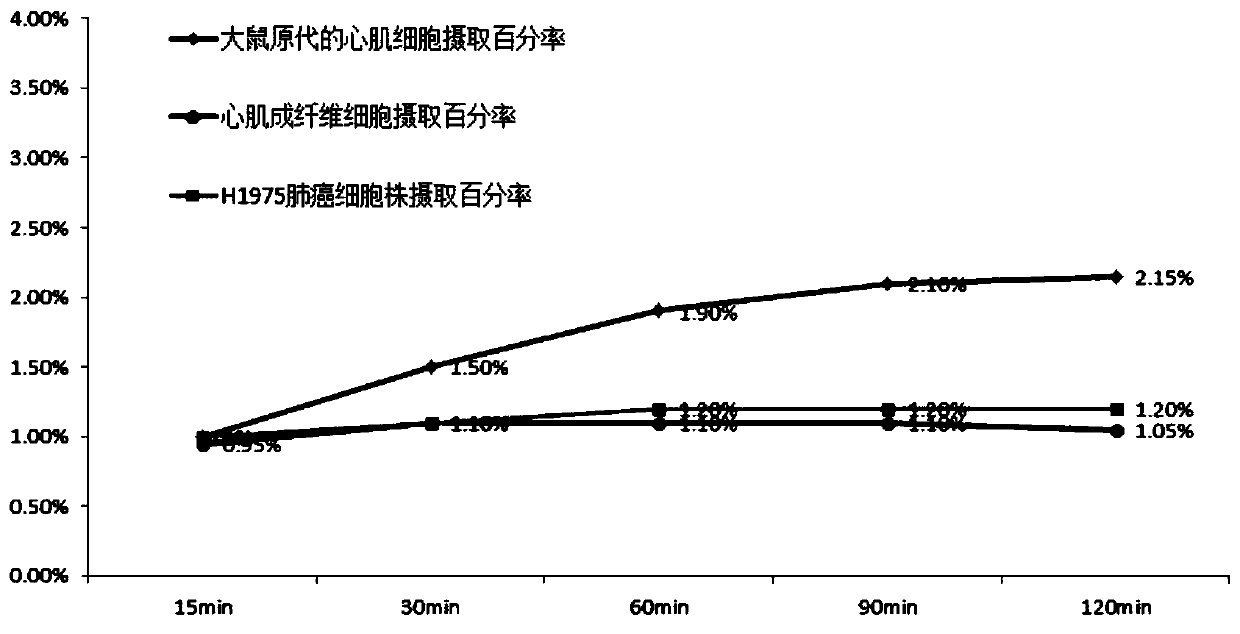
Isoquinolinopyridazinone compound as well as synthesis method and application thereof
A synthesis method and compound technology are applied in the field of isoquinoline pyridazinone compounds and their synthesis, and achieve the effects of easy preparation, easy process optimization, and optimization of drug structure-activity relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0042] Example 1
[0043] A method for synthesizing isoquinolinopyridazinone compounds, including the following steps:
[0044]
[0045] Compound 1N-Boc-4-piperidone (10g, 50mmol) was dissolved in 80ml of dry toluene, added p-toluenesulfonic acid monohydrate (0.3g, 1.5mmol) and tetrahydropyrrole (4.3g, 60mmol), heated Reflux the reaction, separate the water produced by the reaction with an oil-water separator, cool to room temperature after 3h, add p-toluenesulfonic acid monohydrate (0.3g, 1.5mmol) and ethyl glyoxylate (50% in toluene) (11ml , 55mmol), heated to reflux for 2h, cooled to room temperature, the reaction solution was concentrated for about 20ml, 4M hydrochloric acid (54ml) was slowly added dropwise under vigorous stirring, and stirring was continued at room temperature overnight. The organic phase was separated, the aqueous layer was extracted three times with ethyl acetate, the organic layers were combined, washed with saturated brine once, dried over anhydrous sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com