Colorless transparent cordierite microcrystalline glass and preparation method thereof
A technology of glass-ceramic and cordierite, which is applied in the field of colorless and transparent cordierite glass-ceramic and its preparation, can solve the problems of lowering of comprehensive performance, increase of glass melting temperature, change of main crystal phase of glass-ceramic, and the like. The effect of reducing the melting temperature, increasing the thermal expansion coefficient, and good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
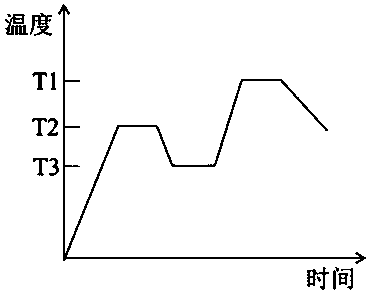
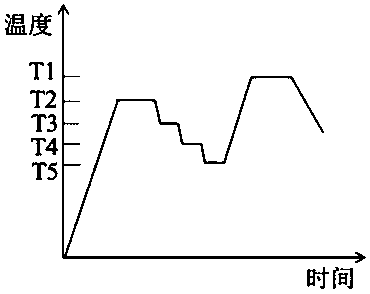
[0037] (1) Magnesium carbonate is used as MgO source, alumina is Al 2 o 3 source, quartz as SiO 2 source, boric acid as B 2 o 3 Source, according to the glass-ceramic molar composition MgO 15.4%, Al 2 o 3 15.4%, SiO 2 65.4%, B 2 o 3 3.8% Calculate the amount of magnesium carbonate, aluminum oxide, quartz and boric acid used. The magnesium carbonate, aluminum oxide, quartz and boric acid are ball milled with ethanol as the medium and dried through an 80-mesh sieve to obtain the batch material. Put the batch material into corundum In the crucible, place it in a silicon-molybdenum rod electric furnace for melting at 1580°C for 3 hours, pour the melted glass melt into a steel mold previously heated to 600°C for molding, and quickly place it in an annealing furnace at 680°C for 5 hours. After cooling to room temperature the base glass was obtained.
[0038] (2) After the base glass is heat-treated according to a certain heat treatment system, it is cooled to room temper...
Embodiment 2
[0045] (1) Use magnesium oxide as the source of MgO and alumina as Al 2 o 3 source, quartz as SiO 2 source, boric acid as B 2 o 3 Source, according to the glass-ceramic molar composition MgO 18%, Al 2 o 3 16%, SiO 2 62.5%, B 2 o 3 3.5% Calculate the amount of magnesia, alumina, quartz and boric acid used, ball mill magnesia, alumina, quartz and boric acid with ethanol as the medium and pass through an 80-mesh sieve to obtain the batch material, and put the batch material into corundum In the crucible, place it in a silicon-molybdenum rod electric furnace for melting at 1550°C for 5 hours, pour the melted glass melt into a steel mold previously heated to 600°C for molding, and quickly place it in an annealing furnace at 650°C for annealing for 3 hours. After cooling to room temperature the base glass was obtained.
[0046] (2) After the base glass is heat-treated according to a certain heat treatment system, it is cooled to room temperature to obtain a colorless tra...
Embodiment 3
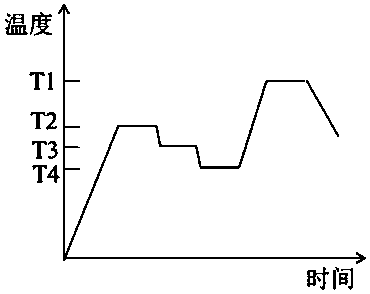
[0051] (1) Use magnesite as MgO source, alumina as Al 2 o 3 source, quartz as SiO 2 source, boric acid as B 2 o 3 Source, in accordance with the glass-ceramic molar composition MgO 15%, Al 2 o 3 10%, SiO 2 72%, B 2 o 3 3% to calculate the amount of magnesite, alumina, quartz and boric acid used, the magnesite, alumina, quartz and boric acid were ball milled with ethanol as the medium and passed through an 80-mesh sieve to dry to obtain the batch materials, and the batch materials were packed Put it into a corundum crucible, put it in a silicon-molybdenum rod electric furnace and melt it at 1650°C for 4 hours, pour the melted glass melt into a steel mold heated to 600°C beforehand, and quickly place it in an annealing furnace at 700°C for annealing After 2h, the base glass was obtained after cooling to room temperature.
[0052] (2) After the base glass is heat-treated according to a certain heat treatment system, it is cooled to room temperature to obtain a colorle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flexural strength | aaaaa | aaaaa |
| flexural strength | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com