PD/graphyne catalyst and its preparation method and application and method for reducing aromatic nitro compounds
A technology of nitro compounds and aromatic nitro groups, applied in the field of biomedicine, can solve the problems of low nitro yield and harsh conditions, and achieve the effects of high reaction yield and overcoming cell drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] This example is used to illustrate the catalyst of the present invention and its preparation method.
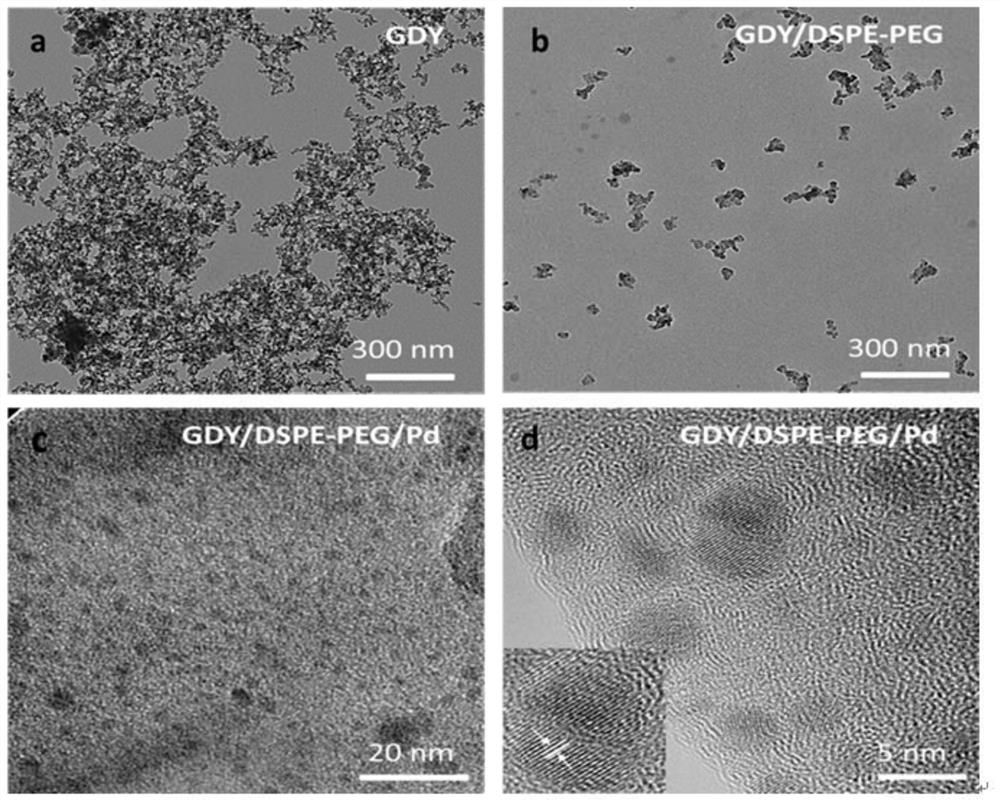
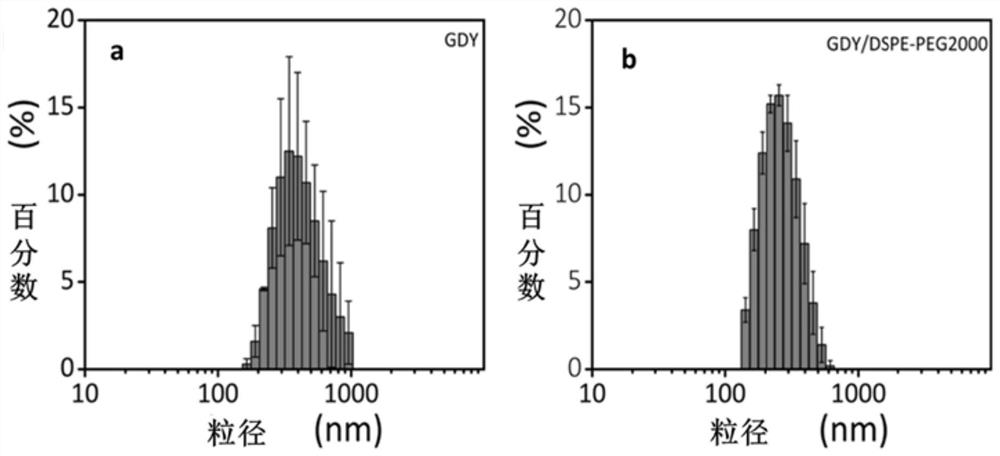
[0080] (1) Add 3.0 mg graphyne (GDY), 100 mg phospholipid-polyethylene glycol (DSPE-PEG, wherein DSPE is 1000 segments, PEG is 2000 segments, and the number average molecular weight is 3000) and 20 mL of water are sequentially added to the beaker , sonicated for 12 hours to make the graphyne dispersed evenly, then ultrafiltration and centrifugation to remove excess DSPE-PEG, and washed three times with ultrapure water to obtain GDY / DSPE-PEG.
[0081] (2) Dissolve GDY / DSPE-PEG in 10mL water, and add K dropwise under ice bath conditions 2 PdCl 4 Aqueous solution (1mL, 30mg / mL), and kept stirring at 0°C for 6h.
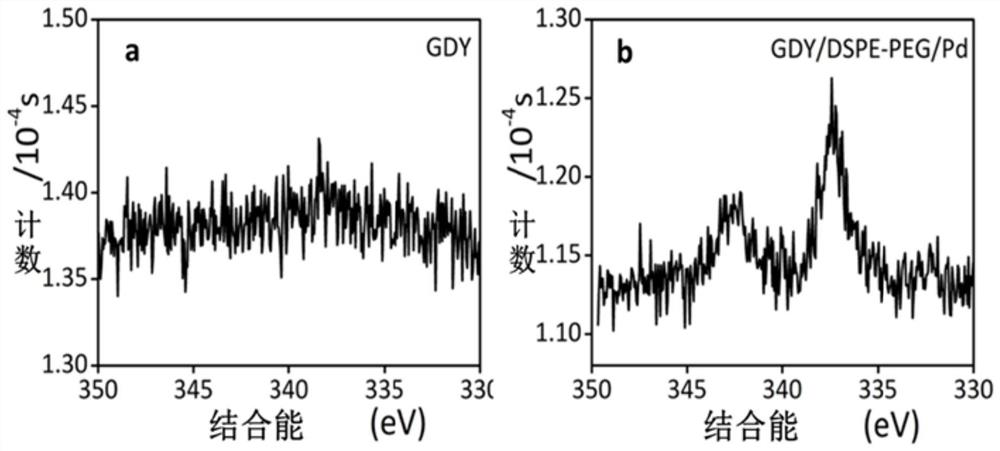
[0082] (3) Centrifuge and wash three times with ultrapure water to remove unreacted K 2 PdCl 4 , freeze-dried to obtain the catalyst GDY / DSPE-PEG / PdGDY / DSPE-PEG / Pd.
[0083] With a transmission electron microscope (such as figure 1 a. figure 1 b. figure 1...
Embodiment 2
[0087] This example is used to illustrate the catalyst of the present invention and its preparation method.
[0088](1) Add 3.0 mg graphyne (GDY), 6 mg polyethylene glycol (PEG, number average molecular weight 3000) and 5 mL water to a beaker in sequence, ultrasonically disperse graphyne for 12 hours, and then ultrafiltration and centrifugation to remove excess PEG, and washed three times with ultrapure water to obtain GDY / PEG.
[0089] (2) Dissolve GDY / PEG in 3mL water, add K dropwise under ice bath condition 2 PdCl 4 Aqueous solution (0.1mL, 30mg / mL), and kept stirring at 0°C for 6h.
[0090] (3) Centrifuge and wash three times with ultrapure water to remove unreacted K 2 PdCl 4 , freeze-dried to obtain the catalyst GDY / PEG / Pd.
[0091] GDY / PEG and GDY / PEG / Pd were characterized by transmission electron microscope, respectively, and it was found that GDY / PEG and GDY / PEG / Pd catalysts were well dispersed in water.
[0092] GDY / PEG was characterized by dynamic light scatte...
Embodiment 3
[0095] This embodiment is used to illustrate catalyst of the present invention and preparation method thereof
[0096] (1) Add 1.0mg graphyne (GDY), 500mg polyarginine (PArg, number average molecular weight 3000) and 50mL water to a beaker in sequence, ultrasonically disperse graphyne for 12 hours, and then ultrafiltration and centrifugation to remove excess Parg, and washed three times with ultrapure water to obtain GDY / PArg.
[0097] (2) Dissolve GDY / PArg in 50mL water, add K dropwise under ice bath condition 2 PdCl 4 Aqueous solution (6.7mL, 30mg / mL), and kept stirring at 0°C for 6h.
[0098] (3) Centrifuge and wash three times with ultrapure water to remove unreacted K 2 PdCl 4 , freeze-dried to obtain the catalyst GDY / PArg / Pd.
[0099] GDY / PArg and Pd / GDY / PArg were characterized by transmission electron microscopy, and it was found that GDY / Parg and GDY / PArg / Pd catalysts were well dispersed in water.
[0100] GDY / PArg was characterized by dynamic light scattering, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com