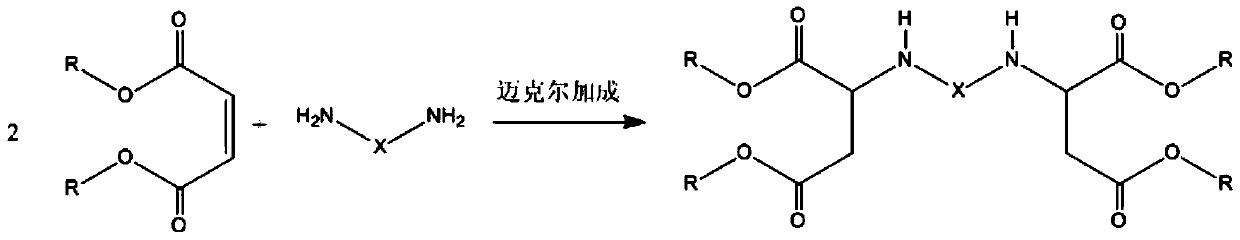
Water-based polyaspartic acid ester resin and preparation method thereof
A technology of aspartic acid ester and resin, which is applied in the direction of polyurea/polyurethane coatings and coatings, can solve the problems of unfavorable coating product construction, over-response, and easy to run out of materials, so as to improve the market competitiveness of products and improve Transesterification conversion rate and the effect of expanding the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
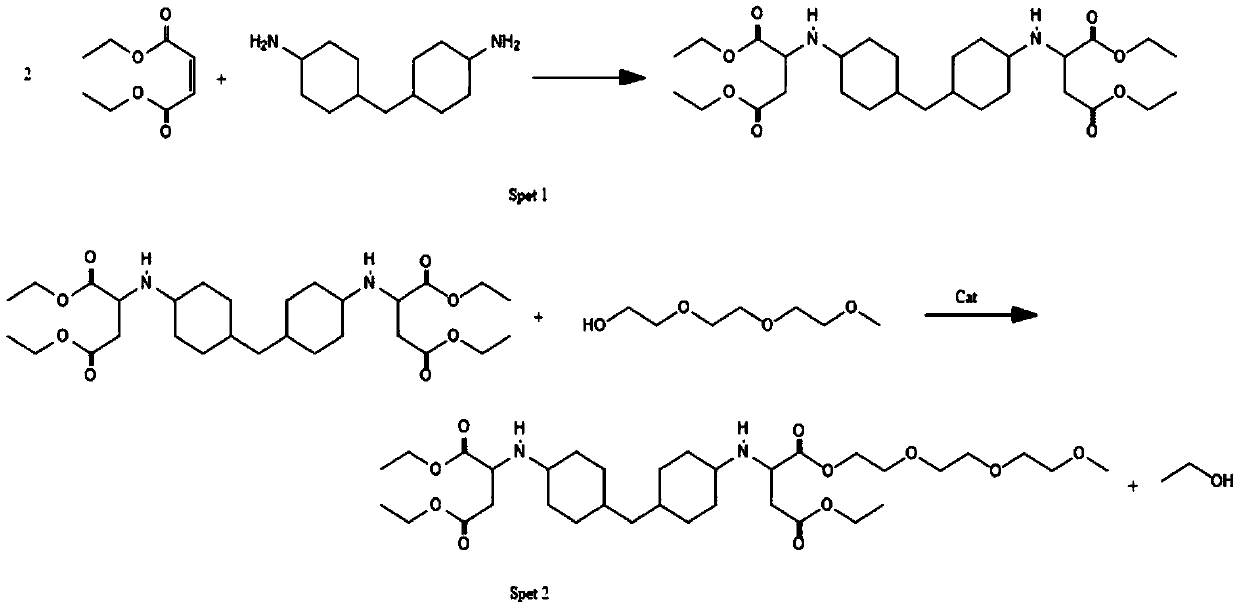
Embodiment 1
[0038] The experimental conditions of Example 1 are: polybasic primary amino compound: maleic acid ester: alkyl etherified monohydroxyl polyether: transesterification catalyst: quencher = 1:2:2:0.01:0.05 (molar ratio) , the polybasic primary amino compound is 4,4'-diaminodicyclohexylmethane, the maleate is diethyl maleate, the alkyl etherified monohydroxy polyether is triethylene glycol monomethyl ether, ester The exchange catalyst is tetra-n-butyl titanate, and the quencher is pure water;
[0039] A kind of preparation method of aqueous polyaspartic acid ester resin, comprises the following steps:
[0040] (1) Addition: Weigh 210g of 4,4'-diaminodicyclohexylmethane into the reaction flask, stir at a speed of 300 rpm, weigh 344g of diethyl maleate into the dropping funnel, Add diethyl maleate dropwise into the reaction bottle through the dropping funnel, control the rate of addition so that the temperature in the reaction bottle is lower than 60°C, and after the addition is c...
Embodiment 2
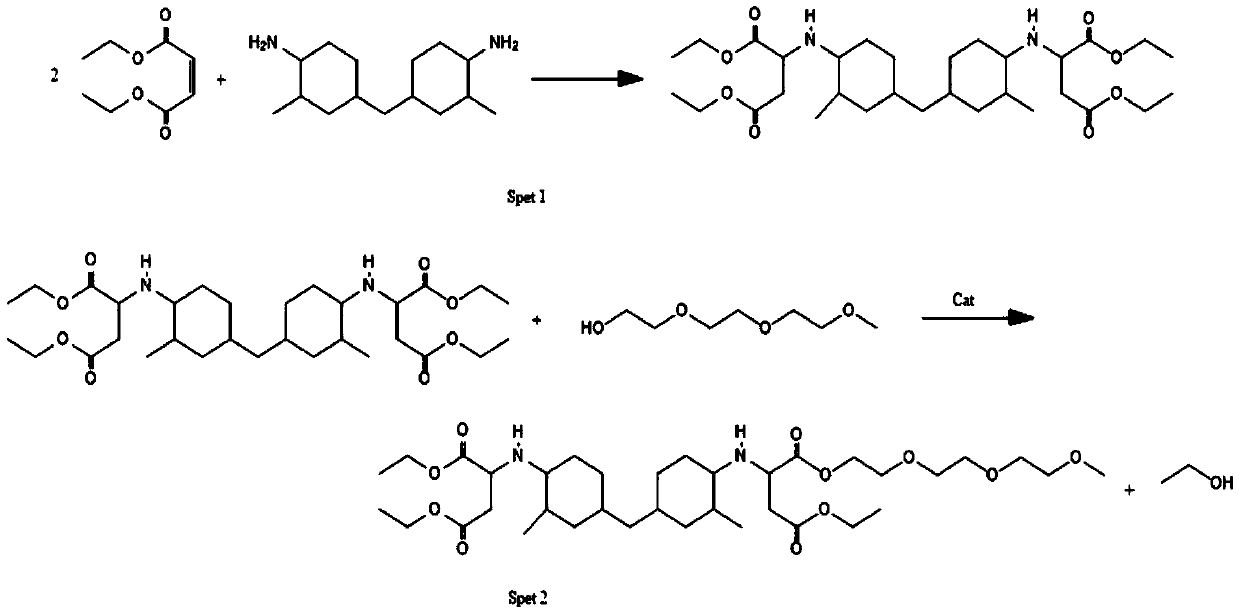
[0046] The difference between Example 2 and Example 1 is that Example 2 uses the same amount of 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane instead of 4,4'-diaminobicyclo Hexylmethane, others are consistent with embodiment 1.
[0047] The chemical reaction process example of embodiment 2 is as follows:
[0048]
Embodiment 3
[0050] The difference between Example 3 and Example 1 is that Example 3 uses the same amount of 2-methyl-1,5-pentanediamine instead of 4,4'-diaminodicyclohexylmethane, and the others are the same as in Example 1 to be consistent.
[0051] The chemical reaction process example of embodiment 3 is as follows:
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com