Application of adinandra nitida and camellianin A in preparation of medicine for treating gastric ulcer
A technology for camellia glycosides and gastric ulcers, applied in the application field of Shibi tea and its active monomers, can solve the problems that there is no relevant research on the therapeutic effect, achieve good therapeutic effect, reduce inflammation, and reduce the degree of injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Shibi tea freeze-dried powder and camelliaside A
[0035] The dry leaves of Shibi tea are crushed with a pulverizer, and the tea powder is extracted in boiling water (the mass-volume ratio of tea / water is 1:20) 3 times, each for 30 minutes. The obtained extracts are filtered and combined, and the extracts are concentrated by rotary evaporation at 60° C., and then freeze-dried into freeze-dried Shibi tea powder for later use.
Embodiment 2
[0037] The preparation method of camellianin A, the inventors disclosed in previous studies: Yuan, E.; Liu, B.; Ning, ZJ Jofp; preservation, Preparation and antioxidant activity of camellianin A from Adinandranitida leaves. 2008, 32(5), 785-797. The method is as follows: Extract 100 g of Shibi tea leaves with 1500 mL of boiling water for 60 minutes. The filtered residue was extracted with 1200 mL boiling water for 60 minutes and filtered. The two filtrates were mixed, stored at 2°C for 24h, and then filtered to collect the precipitate. The precipitate was dried at 60°C for 5 hours to obtain a crude product. The crude product was recrystallized with 20% ethanol for 5 times and purified to obtain crystals. The crystals were dried at 60°C for 3 hours to obtain a light yellow camellin A monomer with a purity of over 98%.
Embodiment 3
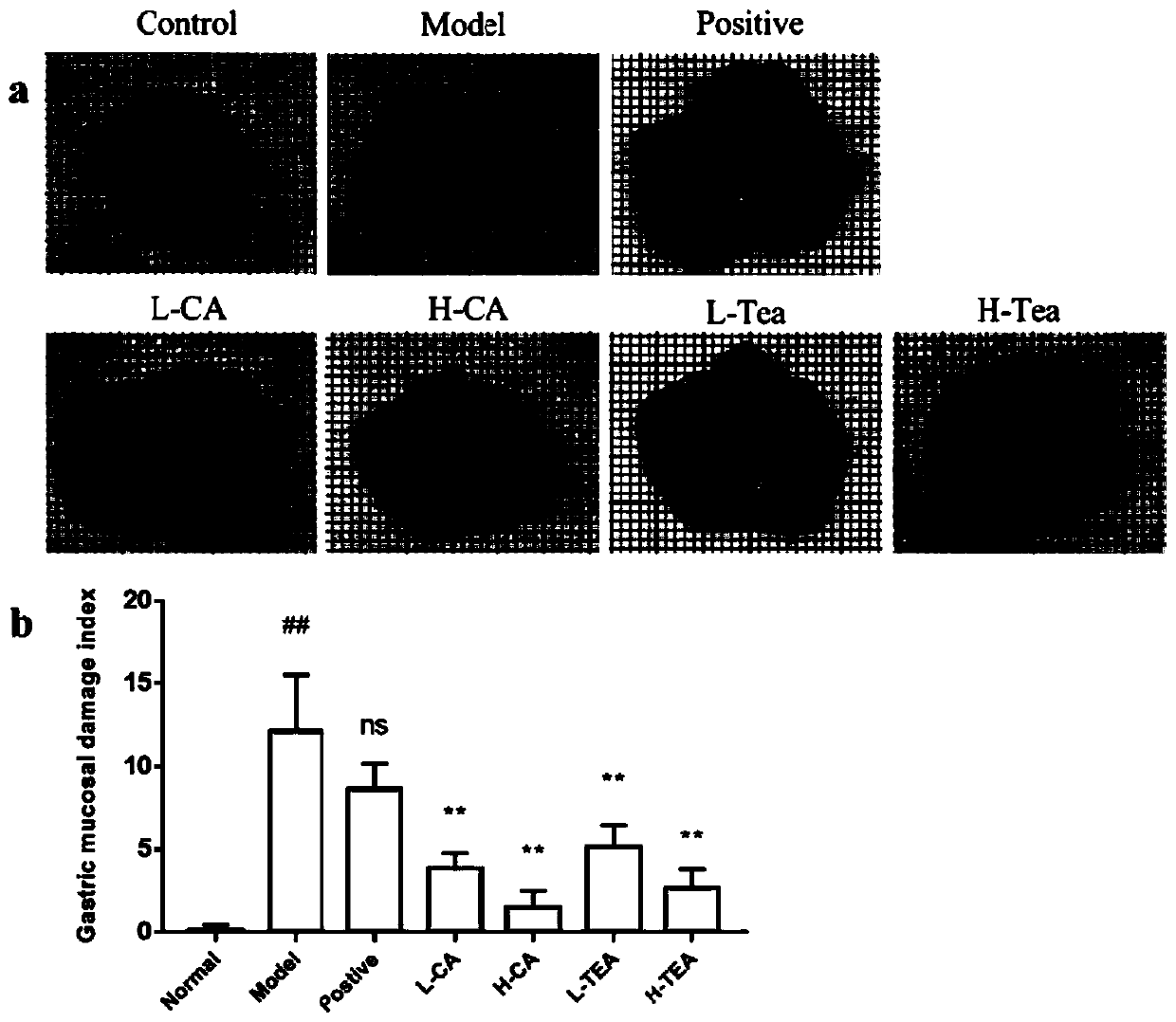
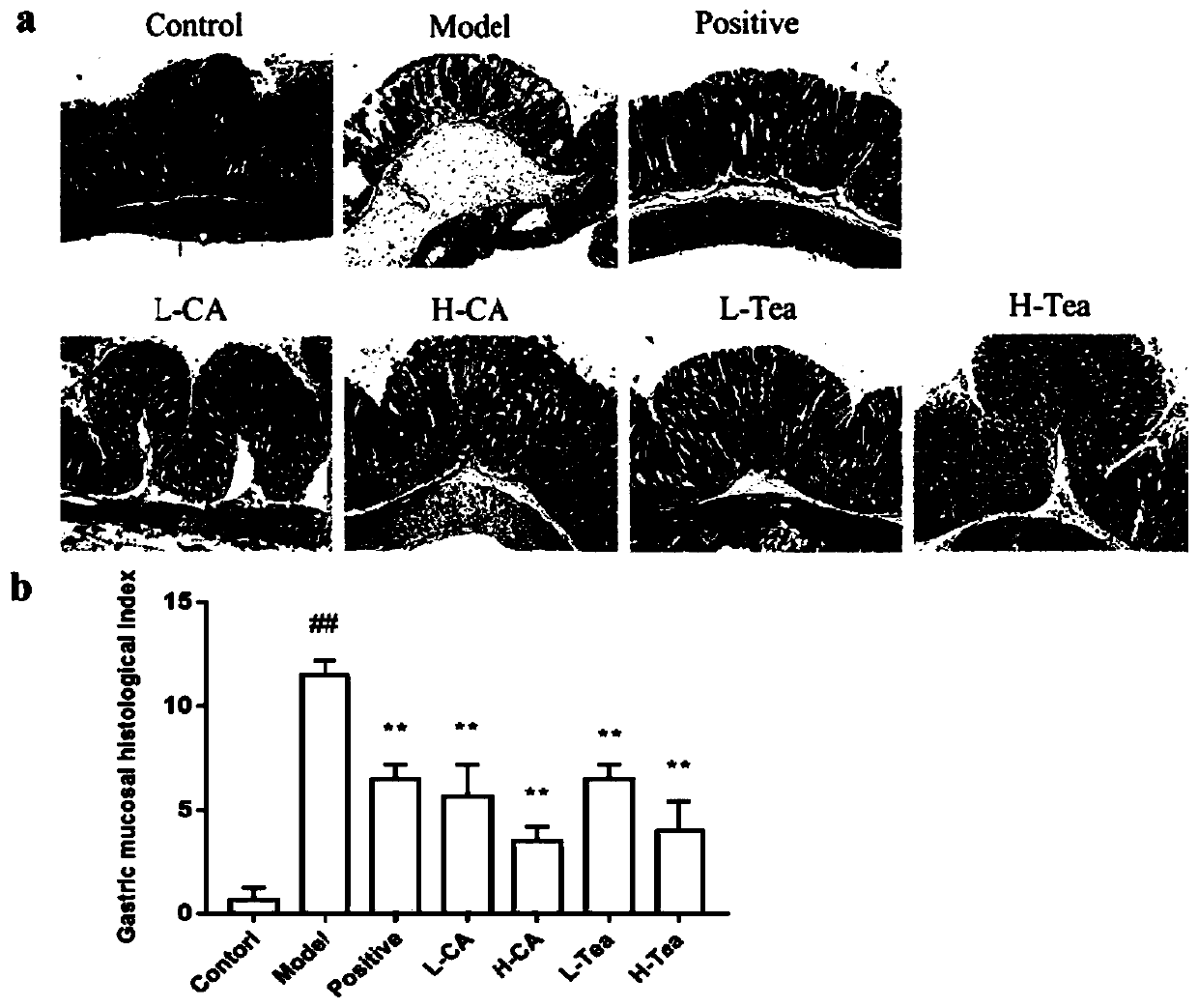
[0039] 1. Construct an animal model of acute gastritis
[0040] Purchasing healthy 7-week-old male ICR mice and rearing them adaptively for 1 week. Randomly group them into: (1) normal control group; (2) model control group; (3) positive drug control group; (4) low-dose camelliaside Group A; (5) High-dose Camellia glycoside A group; (6) Low-dose Shibi tea group; (7) High-dose Shibi tea group, each with 7 animals.
[0041] Except for the normal control group, animals in each group were fasted overnight and then used 60% ethanol + 0.4M hydrochloric acid for acute gastric ulcer modeling. The gastric dose was 10 mL / kg body weight. The animals in the normal control group were fasted overnight and then the same dose Pure water was used as a blank control. After the model was successfully established, the administration was administered for 2 consecutive days the next day.
[0042] Cimetidine, camelliaside A and Shibi tea freeze-dried powder were all dissolved in 0.5% CMCNa solution, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com