Application of zanthoxylum nitidum extract in preparation of medicine for preventing and treating spinal fusion surgery inflammation
A technology of double-sided needles and extracts, applied in the direction of drug combinations, antipyretics, anti-inflammatory agents, etc., can solve the problem of uncontrollable exudation of inflammatory substances, and achieve the effect of reducing high expression and high permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
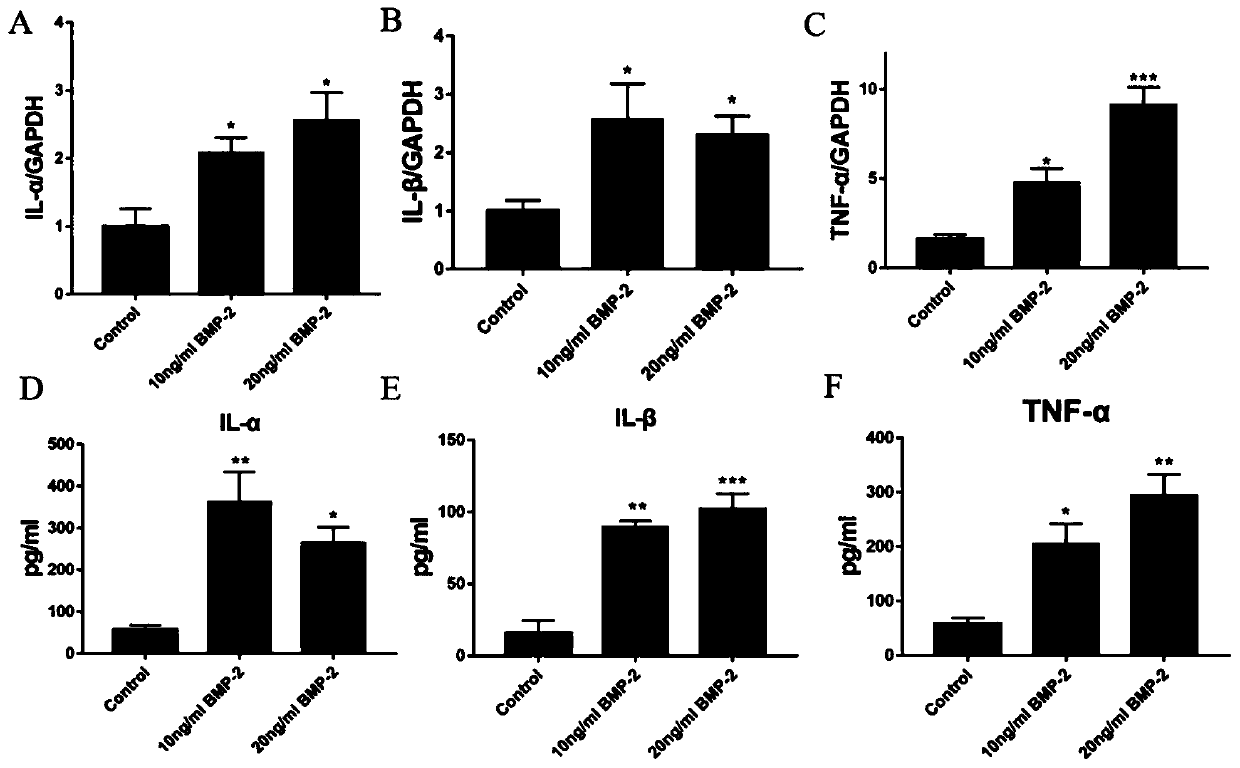
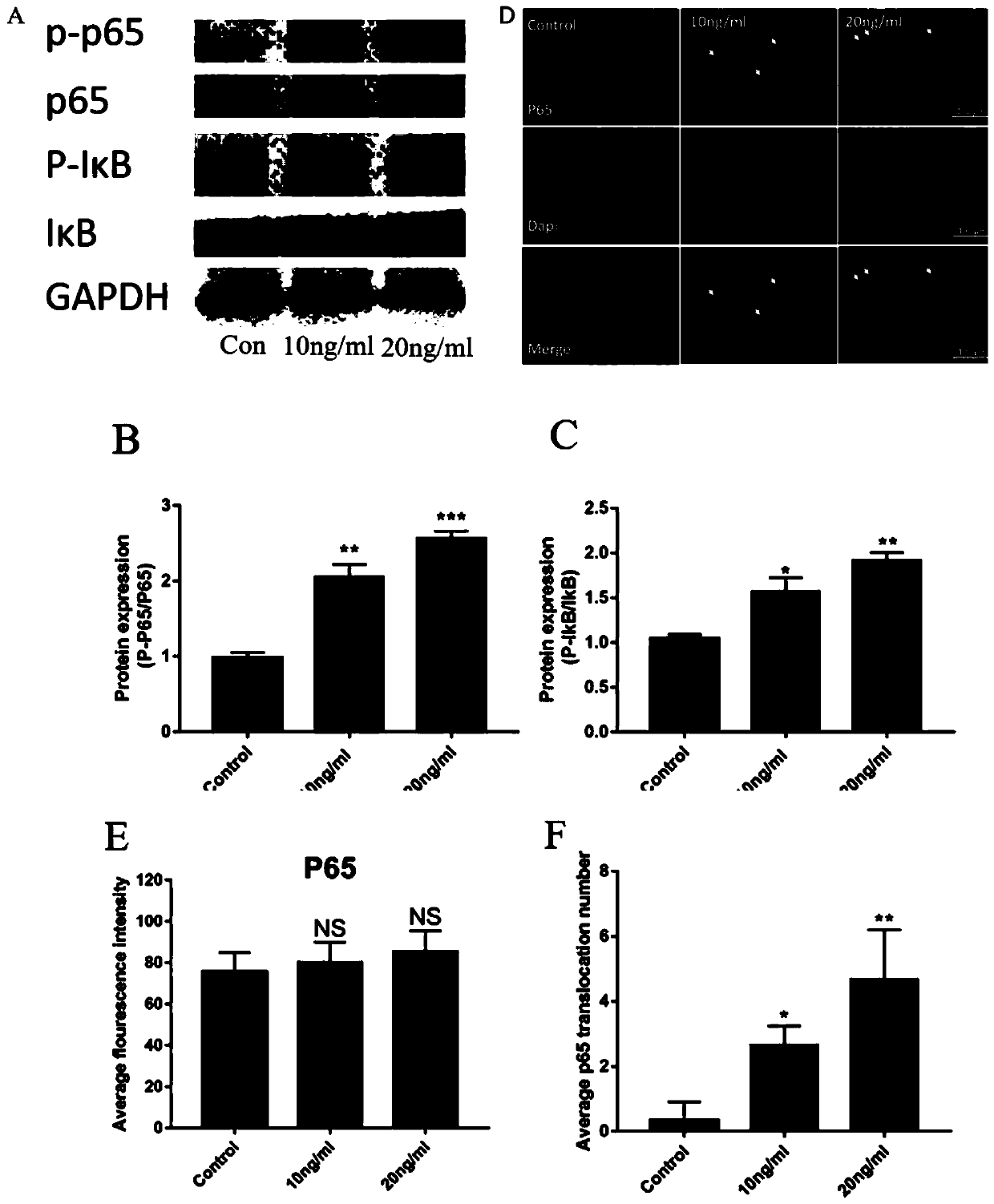
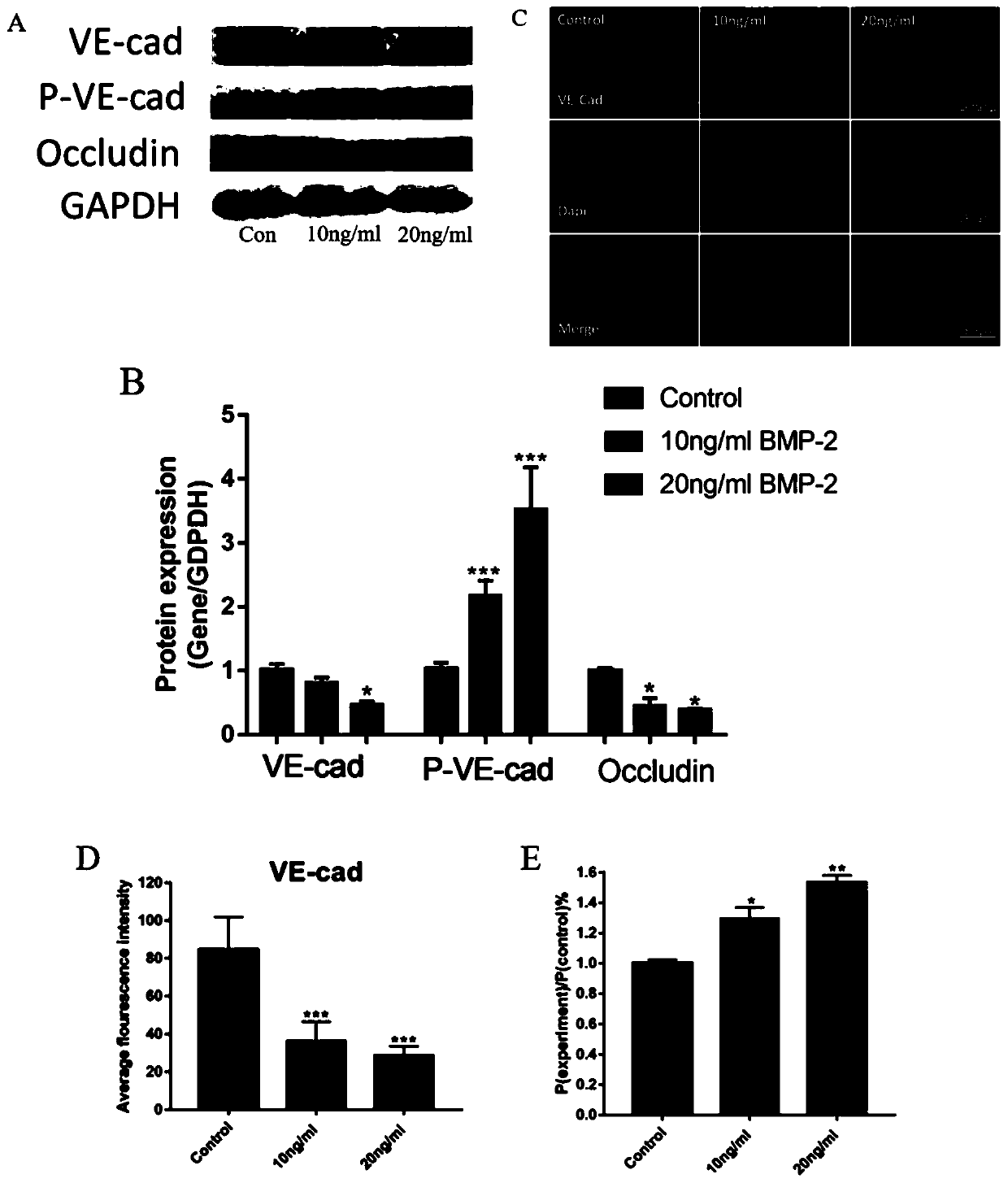
[0030] This example involves the specific implementation process and efficacy verification of the prevention and treatment of BMP-2-induced inflammation around spinal fusion surgery with LMZ extract:
[0031] 1. Cell culture
[0032] Human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection. In high glucose endothelial cell culture medium (Dulbecco's Modified Eagle Medium, Sigma, 211-500) containing 10% fetal bovine serum, 1% penicillin and 1% streptomycin, 37°C, 5% CO 2 cultured in a wet cell incubator. When the whole cell fusion rate reaches about 80%, the drug treatment is carried out.
[0033] 2. Drug handling
[0034] Modeling: RhBMP-2 with a concentration gradient of 0, 10 and 20ng / ml acted on human umbilical vein endothelial cells for 8 hours.
[0035] Intervention with LMZ extract + modeling: 4 hours before 20ng / ml rhBMP-2 intervention, 3 doses of LMZ extract (extractable or commercially available, this example The ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com