Allicin-entrapped cell microparticle drug delivery system as well as preparation method and application thereof
A drug-carrying system and micro-particle technology, applied in the field of medicine, can solve the problems of inability to achieve precise targeted transportation, volatile instability, poor physical and chemical properties of allicin, etc., and achieve high application value and potential, excellent therapeutic effect, significant The effect of anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
[0026] Example 1 Preparation, characterization and drug loading detection of DATS-drug-containing microparticles
[0027] Take 4×10 B16BL6 cells in the logarithmic growth phase 7 cells / mL, irradiated by ultraviolet light for 1 h, added DATS with a concentration of 60 μM, incubated in the incubator for 10 h, collected the cell culture supernatant, centrifuged at 14000 g, 4 °C for 2 min, discarded the precipitate, and took The supernatant was centrifuged at 14,000 g at 4°C for 70 min to obtain DATS-containing microparticle precipitates, namely DATS-drug-containing microparticles. The prepared DATS-drug-containing microparticles were white precipitates at the bottom of the centrifuge tube.
[0028] Take 10 μL of DATS-medicine-containing microparticles, dilute it to 1 mL with pure water, and slowly inject it into the sample pool of the Malvern particle size analyzer, and measure the average particle size, polydispersity index (PDI) and potential of DATS-microparticles . The mea...
Embodiment 2
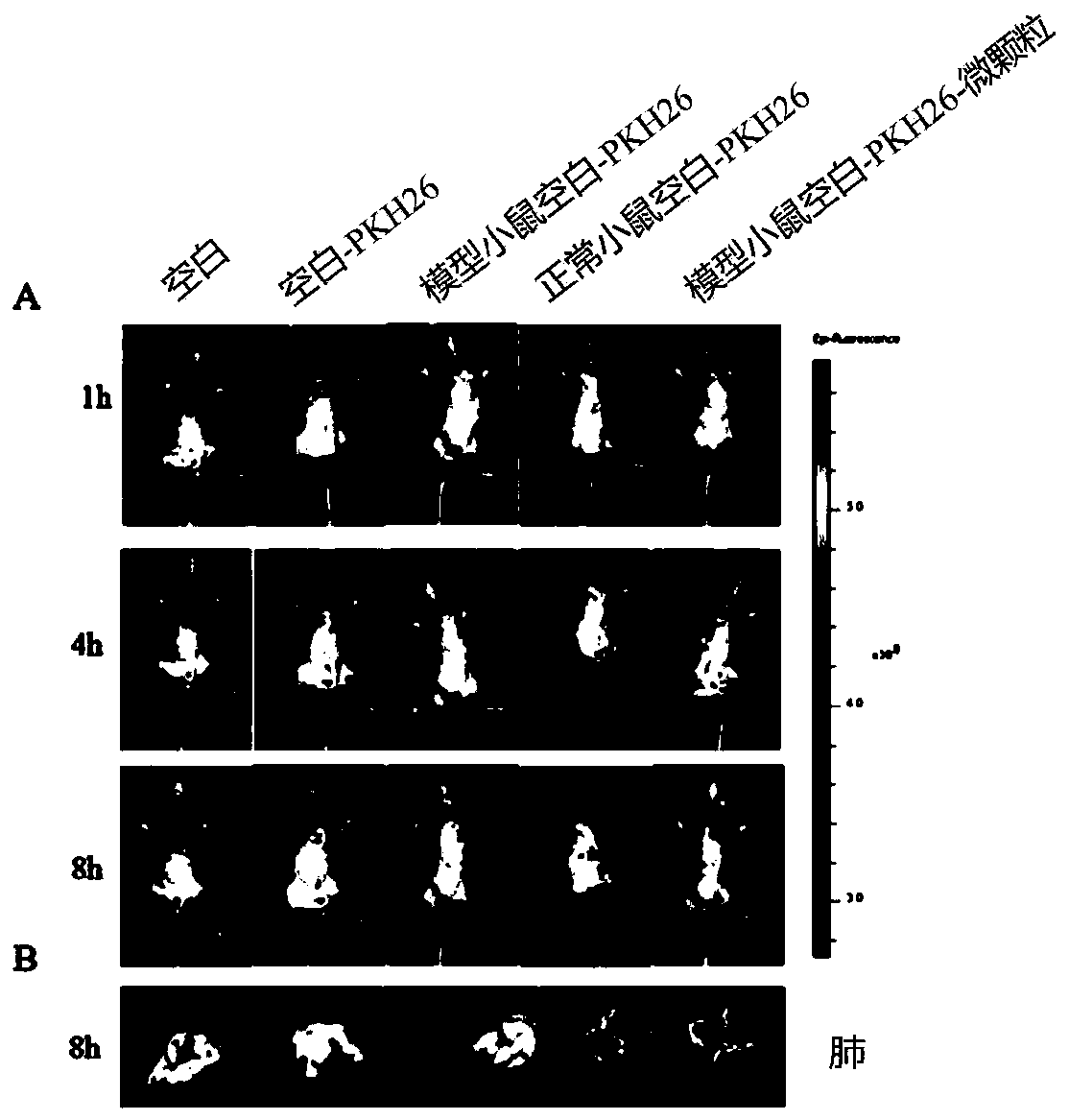
[0035] Example 2 Uptake of DATS-drug-containing cell microparticles by cells and tissue distribution in vivo
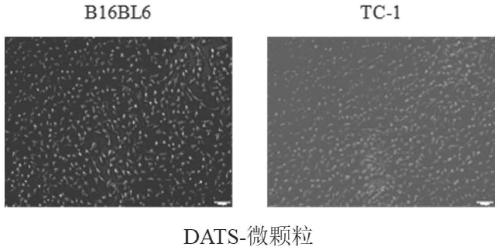
[0036] 1. Study on the uptake of DATS-microparticle drug delivery system by B16BL6 and TC-1 cells
[0037] Blank microparticle extraction: take 4×10 B16BL6 cells in the logarithmic growth phase 7 cells / mL, irradiated with ultraviolet light for 1 h, continued to incubate in the incubator for 10 h, collected the cell culture supernatant, centrifuged at 14000 g, 4 °C for 2 min, discarded the precipitate, and took the supernatant, 14000 g, 4 Centrifuge for 70 min at ℃ to obtain Blank MPs. Preparation of PKH26-DATS-microparticles: Take DATS-microparticles, dilute them to a total protein concentration of 800 ug / mL, take 0.1 mL of DATS-MPs, resuspend in 0.5 mL of DiluentC, record as A, take 2 Add μL of PKH26 to 0.5 mL of DiluentC, denoted as B, then mix A and B, incubate at 25°C for 3 min, then add an equal volume of serum to terminate the reaction, and then centrifuge the...
Embodiment 3
[0043] Example 3 Evaluation of the therapeutic effect of DATS-microparticles on the lung metastasis of mouse melanoma and the intervention of inflammatory microenvironment
[0044] C57BL6 black mice with a body weight of about 18 g were fed adaptively for 7 days and injected with 5×10 5 / mL of B16BL6 melanoma cells, after the mice were inoculated with tumor cells, the mice in each group were randomly divided into model group, blank microparticle group, DATS-microparticle and DATS bare drug group, with 5 mice in each group. Weigh the body weight of the small black mice every day, observe and record the state and death of the animals, start the administration after 23 days, intraperitoneal injection, the administration concentration is 1.8 mg / kg, and administer the administration every other day, after 14 days, the eyeballs are removed to take blood, and the cervical spine is removed The black mice were sacrificed, dissected, and the lung tissues of the mice were taken, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com