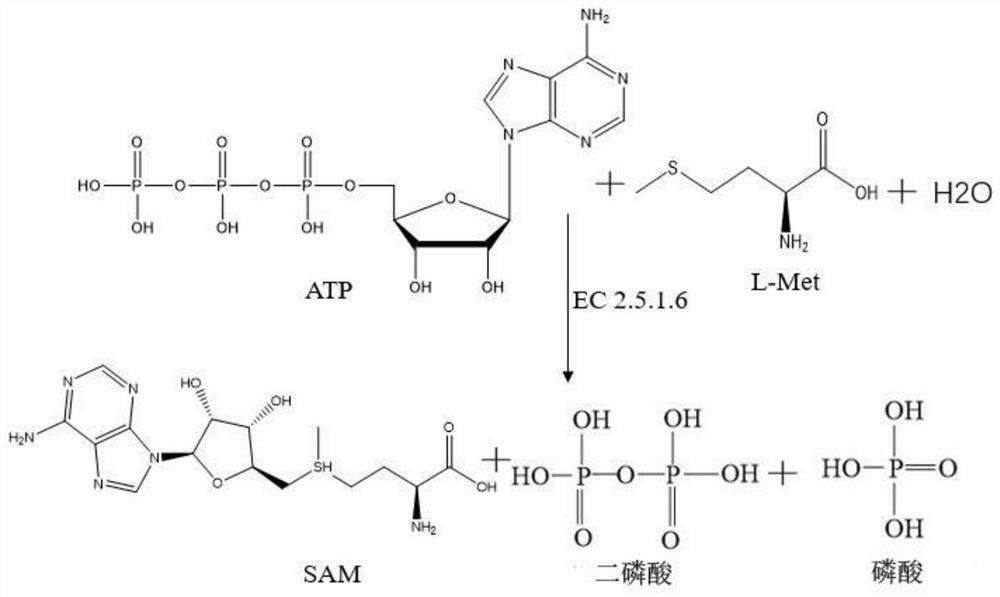
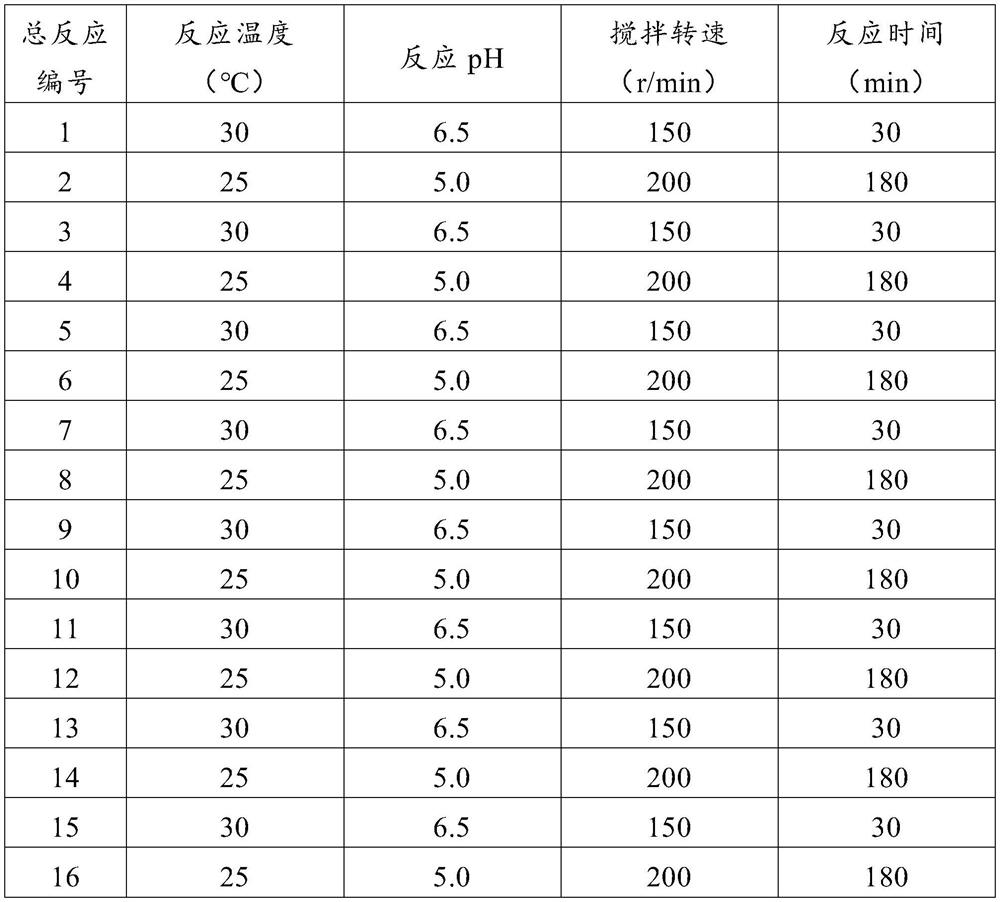
Recombinant Escherichia coli engineering bacteria and its method for preparing s-adenosylmethionine
A technology for recombining Escherichia coli and adenosylmethionine, applied in the field of enzyme engineering, can solve the problems of high cost, easy inactivation, instability of S-adenosylmethionine synthase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Construction of S-adenosylmethionine synthetase expression strain
[0039] 1. Construction of expression strain BL21(DE3) / pET30a-SUMO-MATI
[0040] At the same time, download the amino acid sequence of S-adenosylmethionine synthase MATI derived from rat liver in GenBank (SEQ ID NO.1 in this article, corresponding to GenBank accession number: NP_036992.2) and the small molecule ubiquitin-like modified protein (SUMO ) (SEQ ID NO.12 herein, corresponding to GenBank accession number: AQS95516.1). Link the SUMO amino acid sequence to the N-terminal of the MATI protein amino acid sequence to form a fusion protein sequence, which is provided to Beijing Qingke Biotechnology Co., Ltd. for the whole gene synthesis of the encoding nucleic acid (using E. coli preferred codons). It was constructed into the prokaryotic expression vector pET30a to form a recombinant plasmid vector pET30a-SUMO-MATI. Transform the recombinant plasmid vector pET30a-SUMO-MATI into Escherichia...
Embodiment 2
[0059] Embodiment 2: Construction of ATP synthetase recombinant expression vector
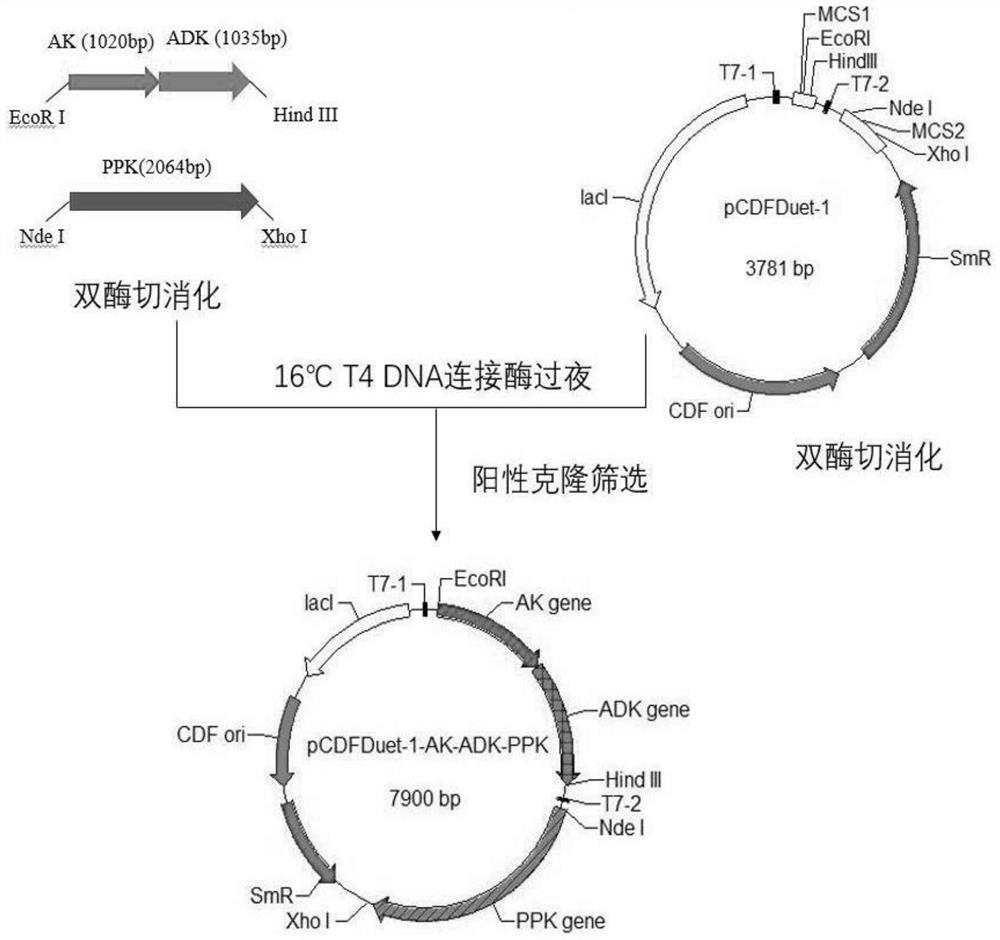
[0060] The amino acid sequence of ATP synthesis-related enzymes (the amino acid sequence of adenosine kinase (EC 2.7.1.20, AK) is shown in SEQ ID NO.9; the amino acid sequence of adenosine kinase (EC 2.7.4.3, ADK) is shown in SEQ ID NO .10; the amino acid sequence of polyphosphate kinase (EC 2.7.4.1, PPK) is shown in SEQ ID NO.11) provided to Beijing Qingke Biotechnology Co., Ltd. for the whole gene synthesis of the encoding nucleic acid (using E. coli preferred codons). Constructed into the prokaryotic expression vector pCDFDuet-1 to form a three-gene co-expression recombinant plasmid vector pCDFDuet-1-AK-ADK-PPK (see the construction flow chart figure 2 ). In pCDFDuet-1-AK-ADK-PPK, the expression genes of adenosine kinase AK and adenylate kinase ADK were constructed into the same reading frame of pCDFDuet-1 to form a double-gene tandem expression, and the expression gene of polyphosphate ki...
Embodiment 3
[0061] Example 3: Construction of S-adenosylmethionine synthetase and ATP synthetase co-expression strain
[0062] Using BL21(DE3) / pET30a-SUMO-MATI as the starting strain, chemically competent cells were prepared, and the three-gene co-expression recombinant plasmid vector pCDFDuet-1-AK-ADK–PPK was transformed into competent cells by using calcium chloride heat shock transformation method Cells BL21(DE3) / pET30a-SUMO-MATI were cultured at 37°C and 220r / min for 60 min, and the transformed cells were coated with kanamycin sulfate (50 μg / mL) and streptomycin ( 25 μg / mL) solid LB medium for positive clone screening, the colony grown on the plate is the co-expression strain BL21(DE3) / pET30a-SUMO of S-adenosylmethionine synthase MATI and ATP synthase -MATI / pCDFDuet-1-AK-ADK-PPK.
[0063] Build in the same way:
[0064] S-adenosylmethionine synthase MATI-1 and ATP synthase co-expression strain BL21(DE3) / pET30a-SUMO-MATI-1 / pCDFDuet-1-AK-ADK-PPK,
[0065] The co-expression strain B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com