Method for synthesizing gamma-butyrolactone containing spiro 1, 3-indandione structure by using micro-channel reaction device
A microchannel reaction, indanedione technology, applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, chemical/physical/physical-chemical processes, etc. Low efficiency and other problems, to achieve the effect of easy control of the reaction process, shortening the reaction time, and speeding up the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
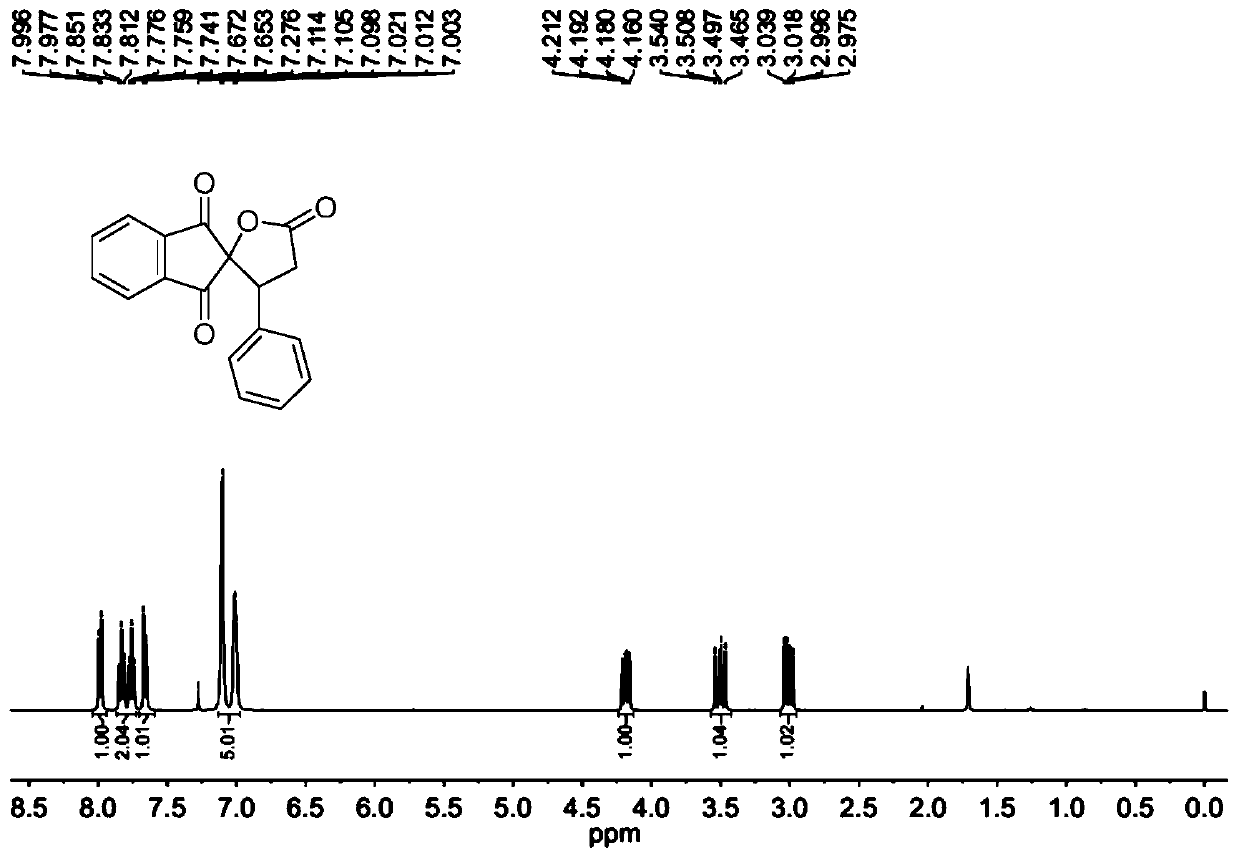
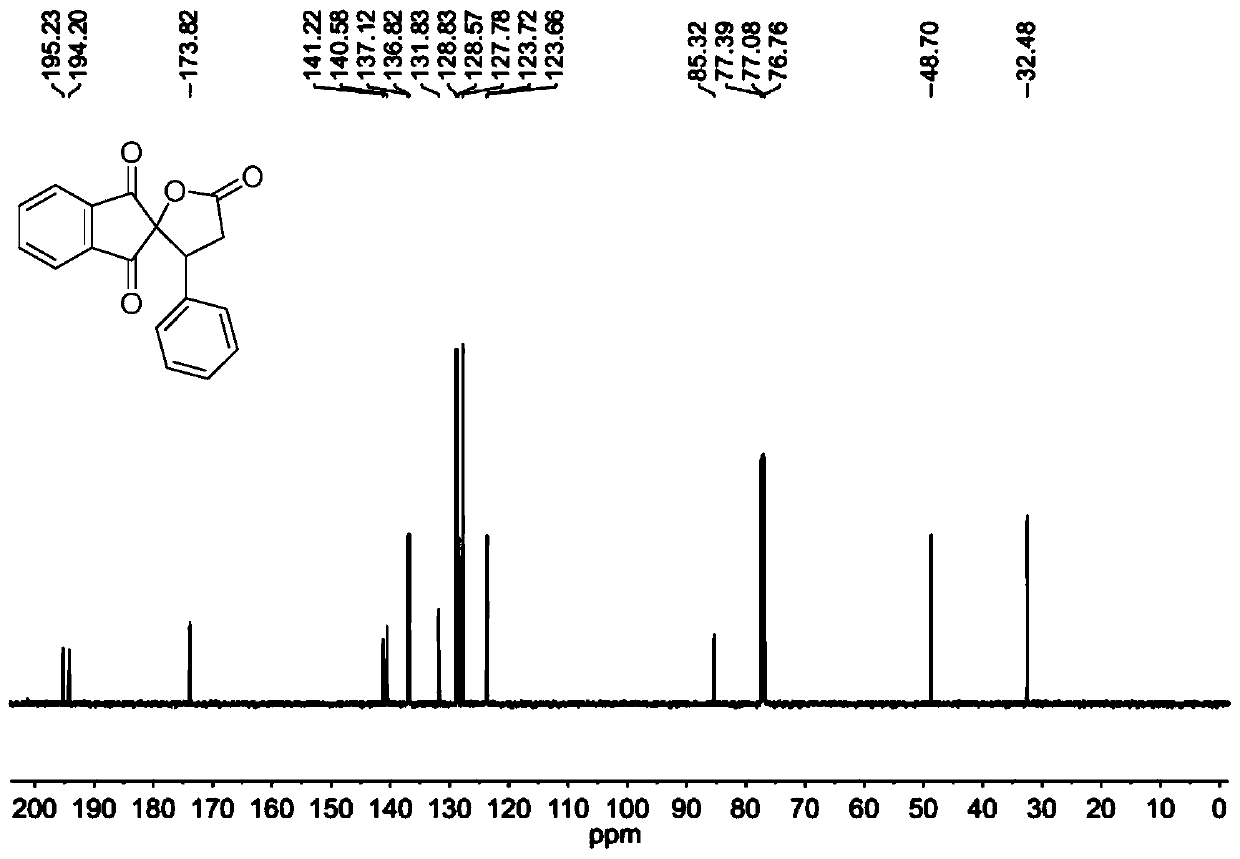
[0078] The molar ratio of 2-benzylidene indene-1,3-dione to cyclo(ethylene)isopropyl malonate is 1:1.5, and the concentration of 2-benzylidene indene-1,3-dione is 0.2mol / L; the molar weight of tetrabutyl ammonium iodide is 20% of 2-benzylidene indene-1,3-dione; the molar weight concentration of tetrabutyl ammonium iodide is 0.04mol / L; 30wt% peroxide The molar amount of the hydrogen solution is three times that of 2-benzylidene indene-1,3-dione, and the ratio of the mixed solution of 1,2-dichloroethane to γ-valerolactone is 1:1.
[0079] Prepare the mixed solution of 2-benzylidene indene-1,3-dione, cyclo(ethylene)isopropyl malonate, 1,2-dichloroethane and γ-valerolactone as solution A; The mixed solution of tetrabutylammonium iodide, 30wt% hydrogen peroxide solution and 1,2-dichloroethane and γ-valerolactone is configured as solution B, and then solution A and solution B are according to the flow volume ratio of 1:1 pumped into the microchannel reaction device, the flow rate ...
Embodiment 2
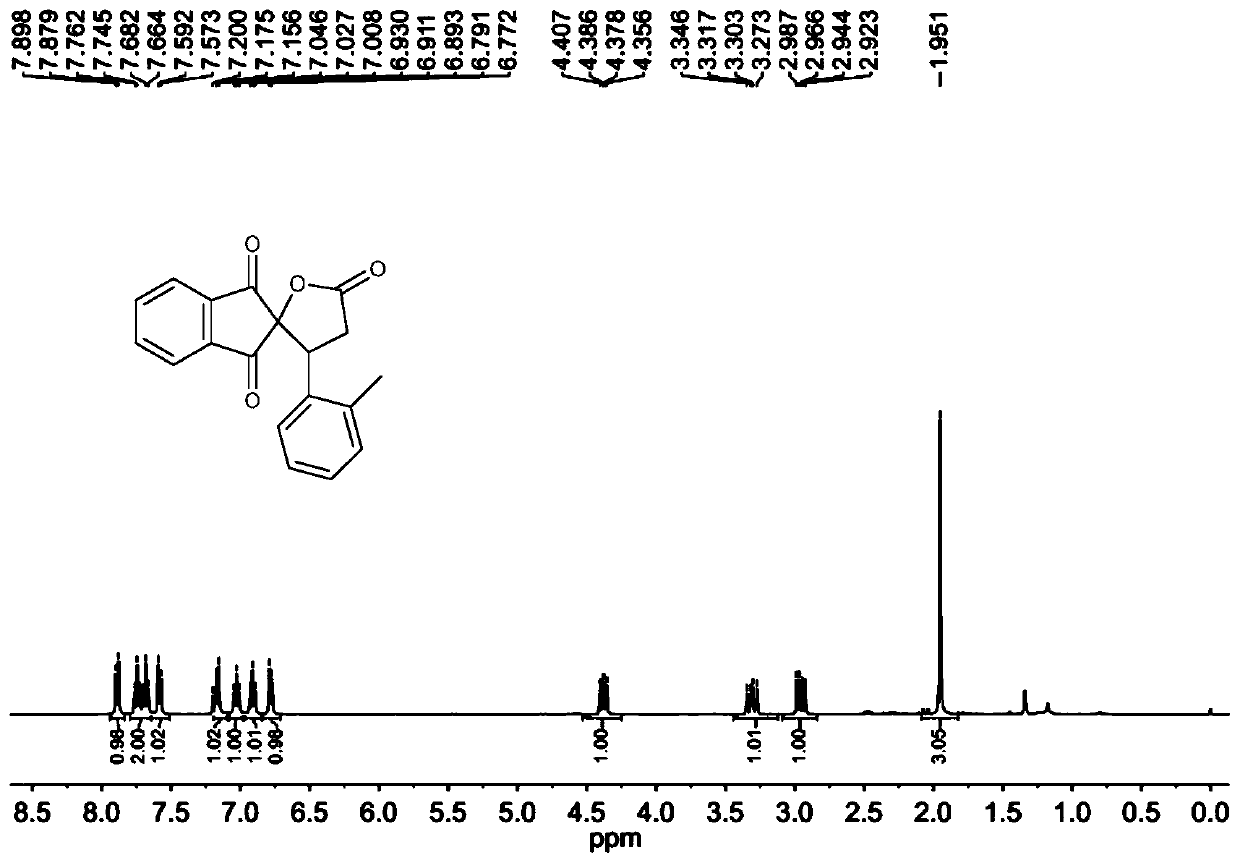
[0097] The molar ratio of 2-[(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione to cyclo(methylene)isopropyl malonate is 1:1.5, 2- The concentration of [(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione is 0.2mol / L; the molar weight of tetrabutylammonium iodide is 2-[(2 20% of -methylphenyl)methylene]-1H-indene-1,3(2H)-dione; the molar concentration of tetrabutylammonium iodide is 0.04mol / L; 30wt% aqueous hydrogen peroxide The molar amount of 2-[(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione is three times that of 2-[(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione, and 1,2-dichloroethane and γ-pentane The mixed solution ratio of ester is 1:1.
[0098] 2-[(2-Methylphenyl)methylene]-1H-indene-1,3(2H)-dione, cyclo(methylene)isopropyl malonate and 1,2-dichloroethane The mixed solution prepared with γ-valerolactone is designated as solution A; the mixed solution obtained by preparing tetrabutylammonium iodide, 30 wt% aqueous hydrogen peroxide and 1,2-dichloroethane and γ-...
Embodiment 3
[0100] The molar ratio of 2-[(2-bromophenyl)methylene]-1H-indene-1,3(2H)-dione to cyclo(ethylene)isopropyl malonate is 1:1.5, 2-[ The concentration of (2-bromophenyl)methylene]-1H-indene-1,3(2H)-dione is 0.2mol / L; the molar amount of tetrabutylammonium iodide is 2-[(2-bromo 20% of phenyl)methylene]-1H-indene-1,3(2H)-dione; the molar concentration of tetrabutylammonium iodide is 0.04mol / L; the molar quantity of 30wt% hydrogen peroxide aqueous solution 3 times that of 2-[(2-bromophenyl)methylene]-1H-indene-1,3(2H)-dione, a mixed solution of 1,2-dichloroethane and γ-valerolactone The ratio is 1:1.
[0101] 2-[(2-Bromophenyl)methylene]-1H-indene-1,3(2H)-dione, cyclo(methylene)isopropylmalonate and 1,2-dichloroethane were mixed with The mixed solution configuration of γ-valerolactone is denoted as solution A; Be solution B, then solution A and solution B are pumped in the microchannel reaction device according to the flow volume ratio of 1:1, and the flow rate is 2.5mL / min, ente...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com