Fluorescent material for sensitively and selectively detecting benzene series as well as preparation method and application thereof
A fluorescent material and reaction technology, applied in the field of fluorescent materials, can solve the problems of slow reaction, complicated operation, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
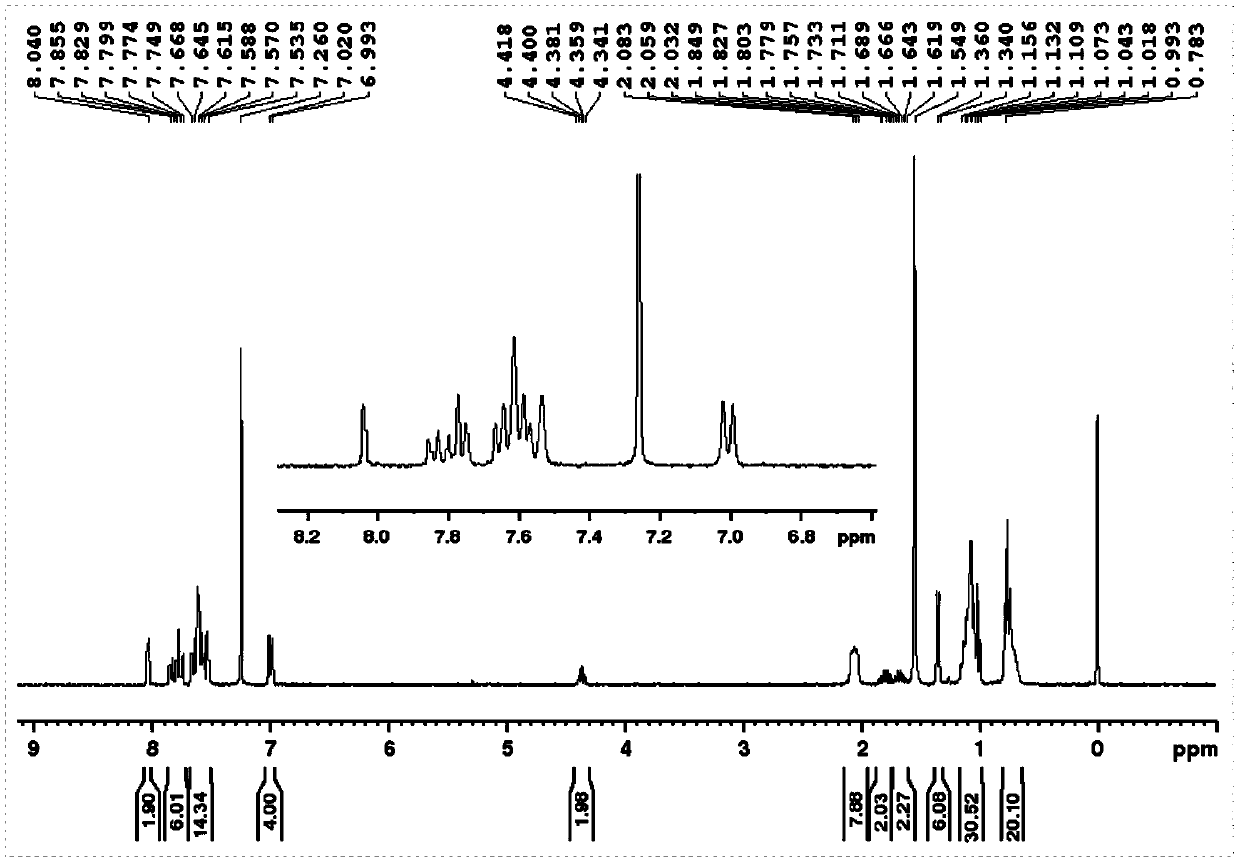
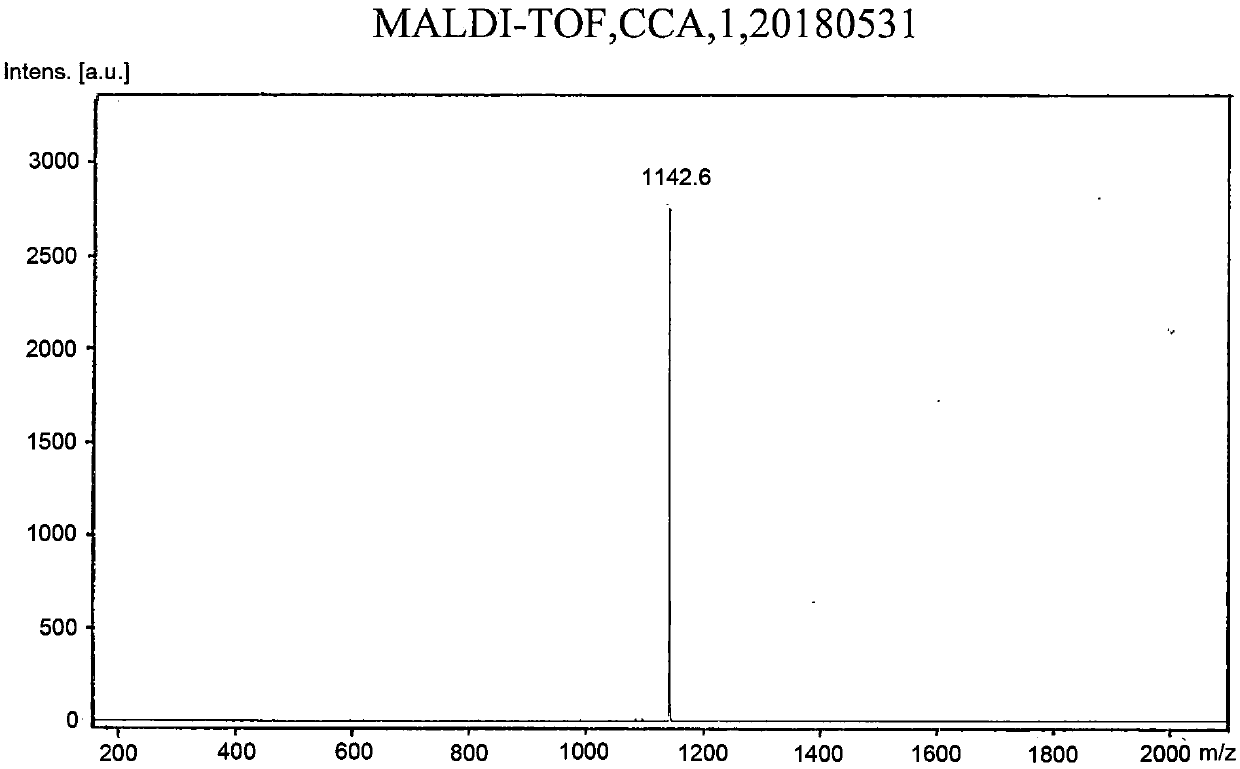
Embodiment 1
[0120] To prepare compound 1,
[0121]
[0122] (1) Add 2 grams of 4-bromophenol and 1 gram of 2-butanol to 30 ml of tetrahydrofuran, add 3.6 grams of triphenylphosphine, put it in an ice bath after deoxygenation, and then slowly add 2.8 grams of it with a needle Diisopropyl azodicarboxylate, stirred at room temperature for 5 hours, filtered through a gel chromatography column to obtain TM-1;
[0123] (2) Get 1.7 grams of the product obtained in step (1), add 2.3 grams of bis-valeryl diboron, 2.2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino)ferrocene dichloride Palladium (II), add 30 ml of 1,4-dioxane, react overnight at 80 degrees Celsius after deoxygenation, and obtain TM-2 after filtering through a gel chromatography column;
[0124] (3) 1.1 grams of the product obtained in step (2), 2.3 grams of 9,9-dihexyl-2,7-dibromofluorene, 0.3 grams of tetrakistriphenylphosphine palladium, 1.7 grams of potassium carbonate, and then 20 ml of 1,4-dioxane a...
Embodiment 2
[0137] To prepare compound 2,
[0138]
[0139]
[0140] (1) Add 2 grams of 4-bromophenol and 1 gram of 2-butanol to 30 ml of tetrahydrofuran, add 3.6 grams of triphenylphosphine, put it in an ice bath after deoxygenation, and then slowly add 2.8 grams of it with a needle Diisopropyl azodicarboxylate, stirred at room temperature for 5 hours, filtered through a gel chromatography column to obtain TM-1;
[0141] (2) Get 1.7 grams of the product obtained in step (1), add 2.3 grams of bis-valeryl diboron, 2.2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino)ferrocene dichloride Palladium (II), add 30 ml of 1,4-dioxane, react overnight at 80 degrees Celsius after deoxygenation, and obtain TM-2 after filtering through a gel chromatography column;
[0142] (3) 1.1 grams of the product obtained in step (2), 2.3 grams of 9,9-dihexyl-2,7-dibromofluorene, 0.3 grams of tetrakistriphenylphosphine palladium, 1.7 grams of potassium carbonate, and then 20 ml of 1...
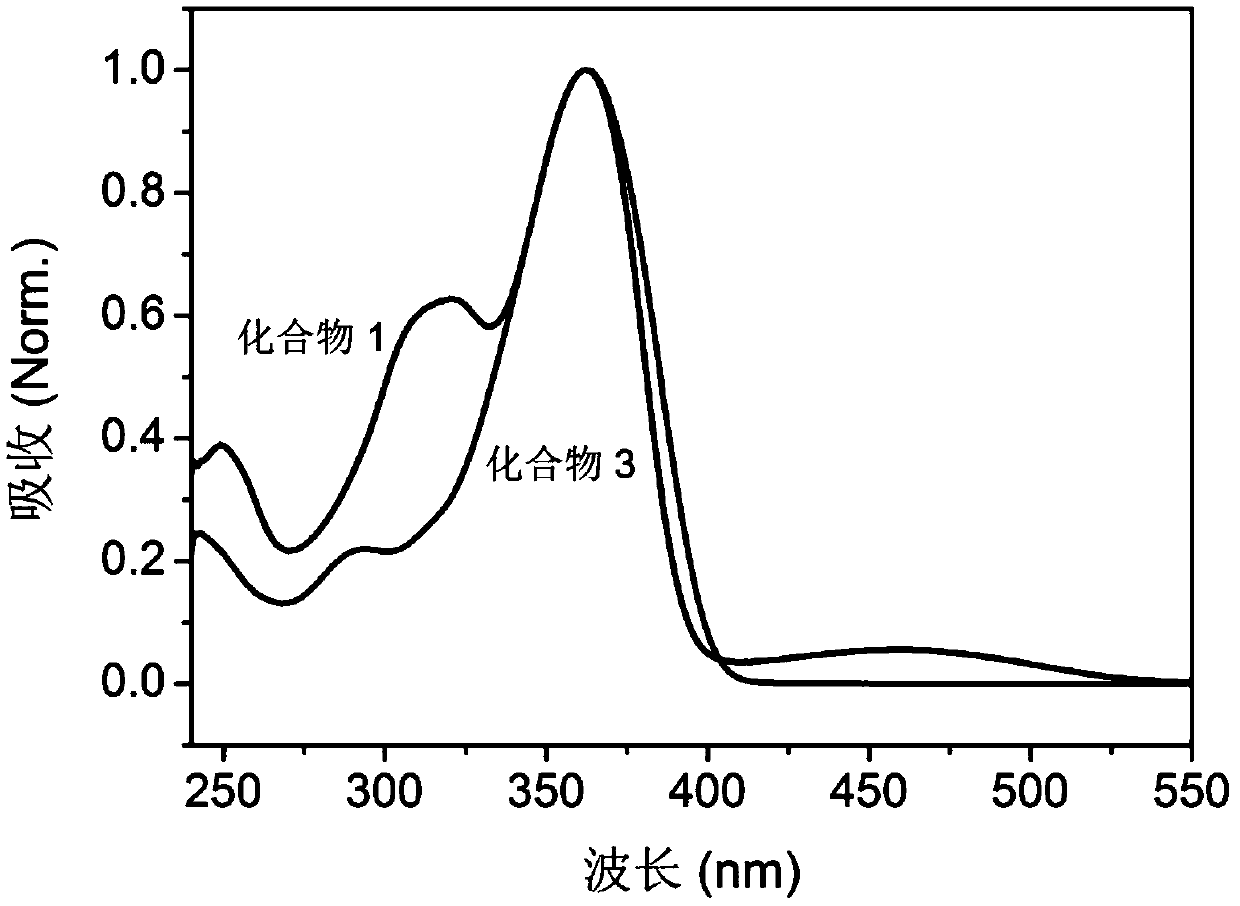
Embodiment 3
[0150] Compound 3 having the following molecular formula was prepared.
[0151]
[0152] (1) Add 2 grams of 4-bromophenol and 1 gram of 2-butanol to 30 ml of tetrahydrofuran, add 3.6 grams of triphenylphosphine, put it in an ice bath after deoxygenation, and then slowly add 2.8 grams of it with a needle Diisopropyl azodicarboxylate, stirred at room temperature for 5 hours, filtered through a gel chromatography column to obtain TM-1;
[0153] (2) Get 1.7 grams of the product obtained in step (1), add 2.3 grams of dipivaloyl diboron, 2.2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino)ferrocene dichloride Palladium(II), add 30 ml of 1,4-dioxane, react overnight at 80 degrees Celsius after deoxygenation, and obtain TM-2 after filtering through a gel chromatography column;
[0154](3) 1.1 grams of the product obtained in step (2), 2.3 grams of 9,9-dihexyl-2,7-dibromofluorene, 0.3 grams of tetrakistriphenylphosphine palladium, 1.7 grams of potassium carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com