Fluorescent probe and preparation method and application thereof
A technology of fluorescent probes and probes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex synthesis, poor water solubility, insufficient selectivity and sensitivity, etc., and achieve high luminous intensity and stable performance , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Dissolve compound A (0.76g, 2.12mmol) and 4-pyridineacetonitrile (0.25g, 2.12mmol) in absolute ethanol (10mL), then add piperidine (0.1mL) to the reaction solution, and reflux at 80°C React for 5 hours. After the reaction was finished, the filtrate was removed by suction filtration, and the filter cake was retained, purified by column chromatography, and the eluent was dichloromethane / ethyl acetate (1:1, volume ratio), and spin-dried under reduced pressure to obtain 0.838 g of a yellow solid ( The yield was 86%). After the product is analyzed by proton nuclear magnetic resonance spectrum, its structural formula is as shown in formula (1):
[0042]
[0043] Wherein the structural formula of compound A is as shown in formula (2):
[0044]
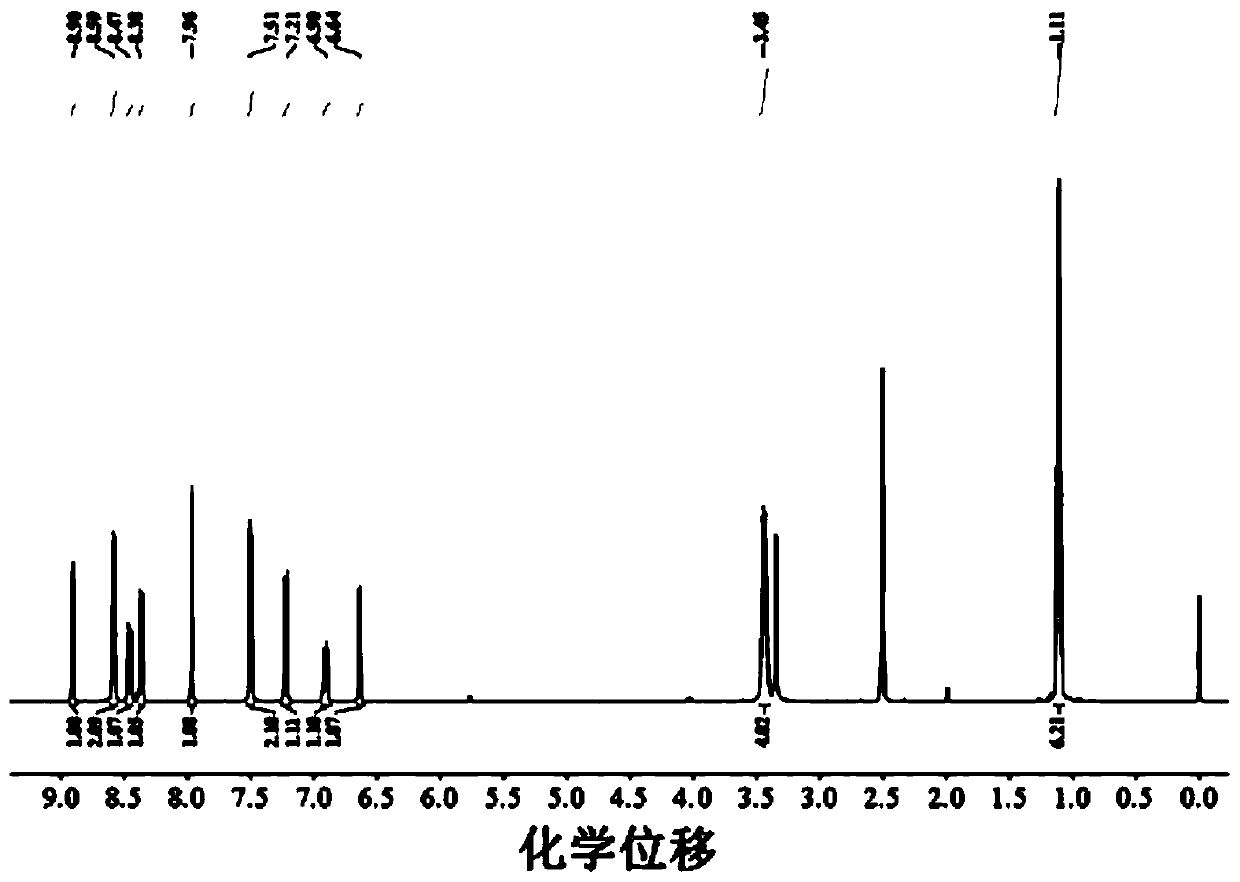
[0045] The H NMR spectrum is as figure 1 As shown, the description of the spectrum is as follows:
[0046] 1 H NMR (400Hz, CDCl 3 ): 1 H NMR (400MHz, CDCl 3): δ8.96(s,1H), 8.71(s,2H), 8.54(s,1H), 8.11(s,1H), 7.51(s,2H), 7...
Embodiment 2
[0048] Compound A (0.76g, 2.12mmol) and 4-pyridineacetonitrile (0.25g, 2.12mmol) were dissolved in anhydrous acetonitrile (10mL), then triethylamine (0.1mL) was added to the reaction solution, and at 50°C Reflux for 5 hours. After the reaction was finished, the filtrate was removed by suction filtration, and the filter cake was retained, purified by column chromatography, and the eluent was dichloromethane / ethyl acetate (1:1, volume ratio), and spin-dried under reduced pressure to obtain 0.563 g of a yellow solid ( Yield 57%).
Embodiment 3
[0050] Dissolve compound A (0.76g, 2.12mmol) and 4-pyridineacetonitrile (0.25g, 2.12mmol) in absolute ethanol (10mL), then add sodium acetate (0.1mL) to the reaction solution, and reflux at 80°C React for 12 hours. After the reaction was finished, the filtrate was removed by suction filtration, and the filter cake was retained, purified by column chromatography, and the eluent was dichloromethane / ethyl acetate (1:1, volume ratio), and spin-dried under reduced pressure to obtain 0.746 g of a yellow solid ( Yield 76%).
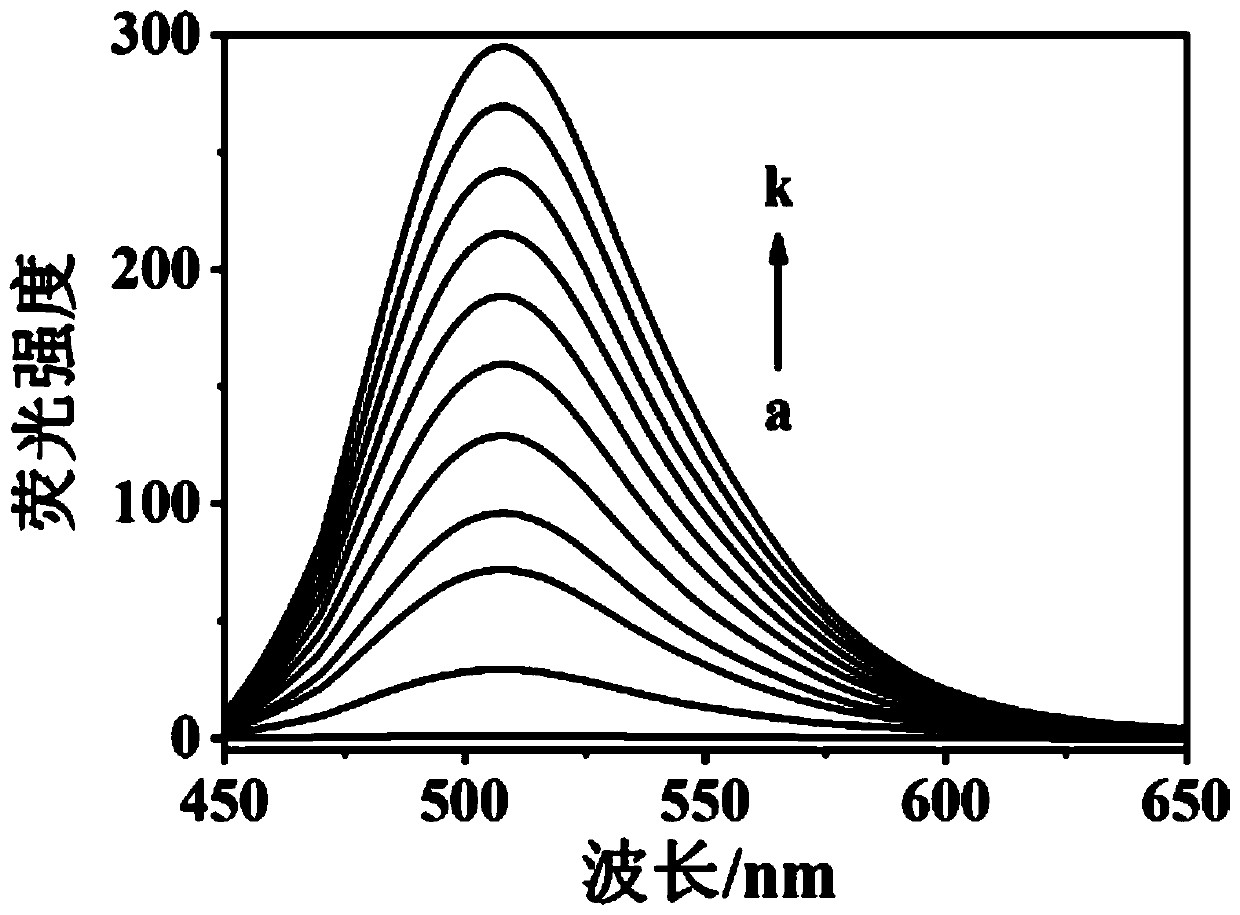
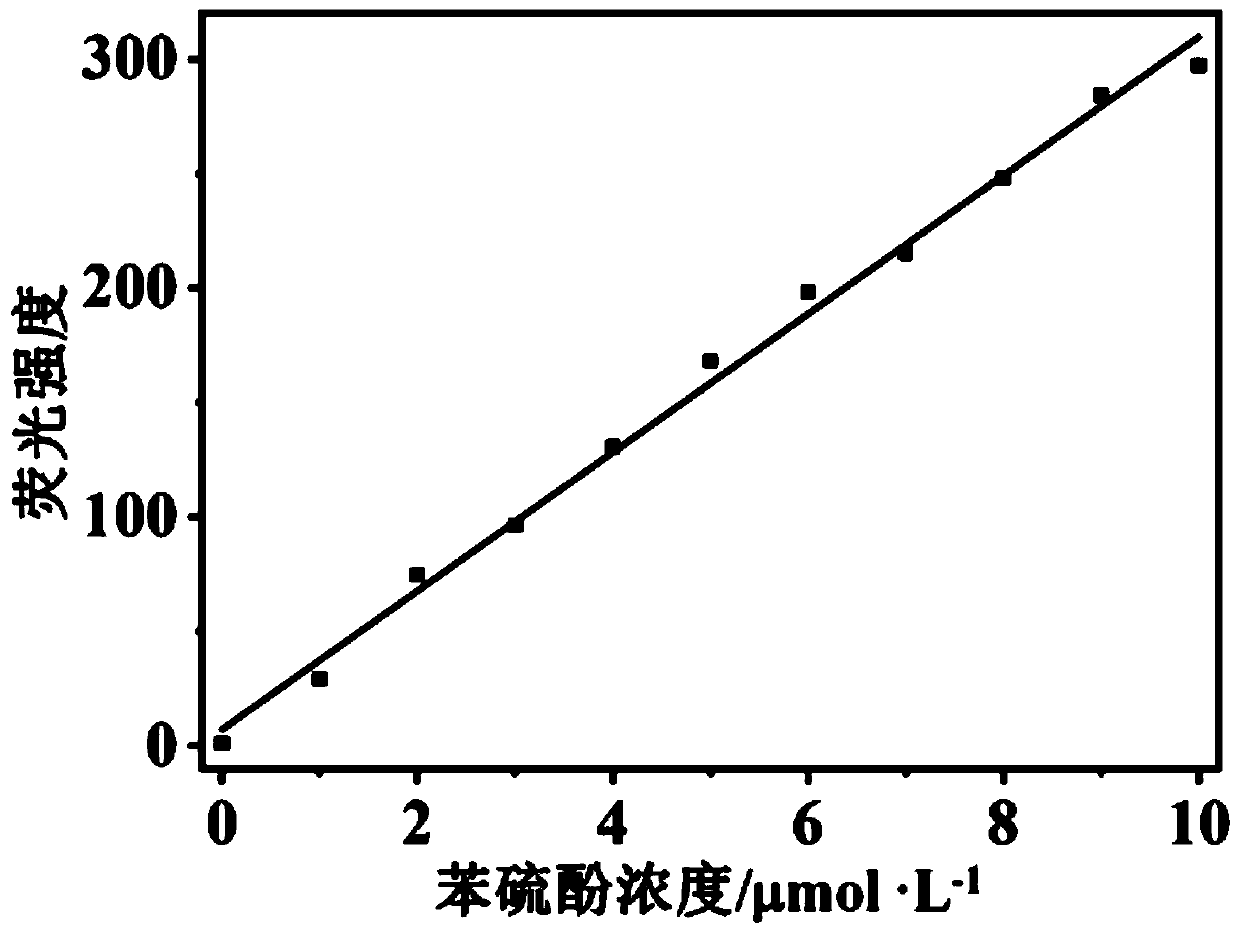
[0051] Fluorescent detection of thiophenol with fluorescent probes:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com