Application of mesenchymal stem cells in preparation of products for treating rheumatoid arthritis
A mesenchymal stem cell and rheumatoid technology, which can be applied to the application field of mesenchymal stem cells in the preparation of products for the treatment of rheumatoid arthritis, and can solve the problem that the pathogenesis is not very clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1. Preparation of mesenchymal stem cell preparations from Wharton's umbilical cord
[0060] 1. Obtaining the umbilical cord of the fetus
[0061] Take the isolated umbilical cord of a full-term newborn without congenital disease; the parturient does not have infectious diseases such as hepatitis, syphilis, AIDS, and the parturient and family members have informed consent to the use of the umbilical cord for experimental research.
[0062] 2. Obtaining mesenchymal cells in the Wharton area of the umbilical cord
[0063] 1. Pretreatment of umbilical cord
[0064] In a sterile laboratory bench, the umbilical cord is repeatedly rinsed with normal saline to wash away residual blood. Cut the umbilical cord into 2-3cm sections with sterile surgical instruments, cut the umbilical cord longitudinally, remove the umbilical artery, umbilical vein and amniotic membrane, take the Wharton area and cut it into 0.5-1mm 3 Small pieces around.
[0065] 2. Obtaining primary umbilical cord...
Embodiment 2
[0077] Example 2. Identification of P3 cell preparation
[0078] The test product is the P3 cell preparation prepared in Example 1.
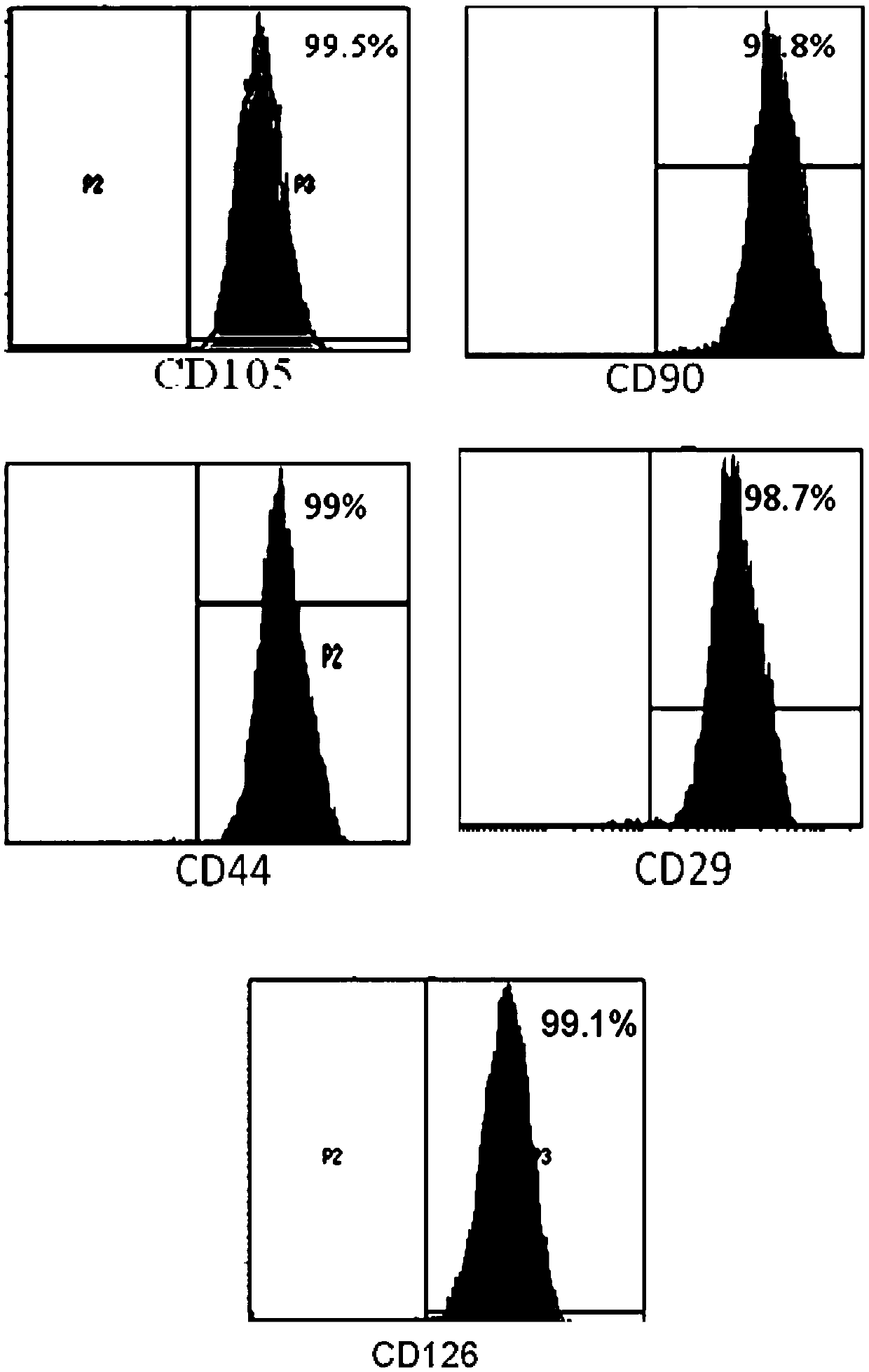
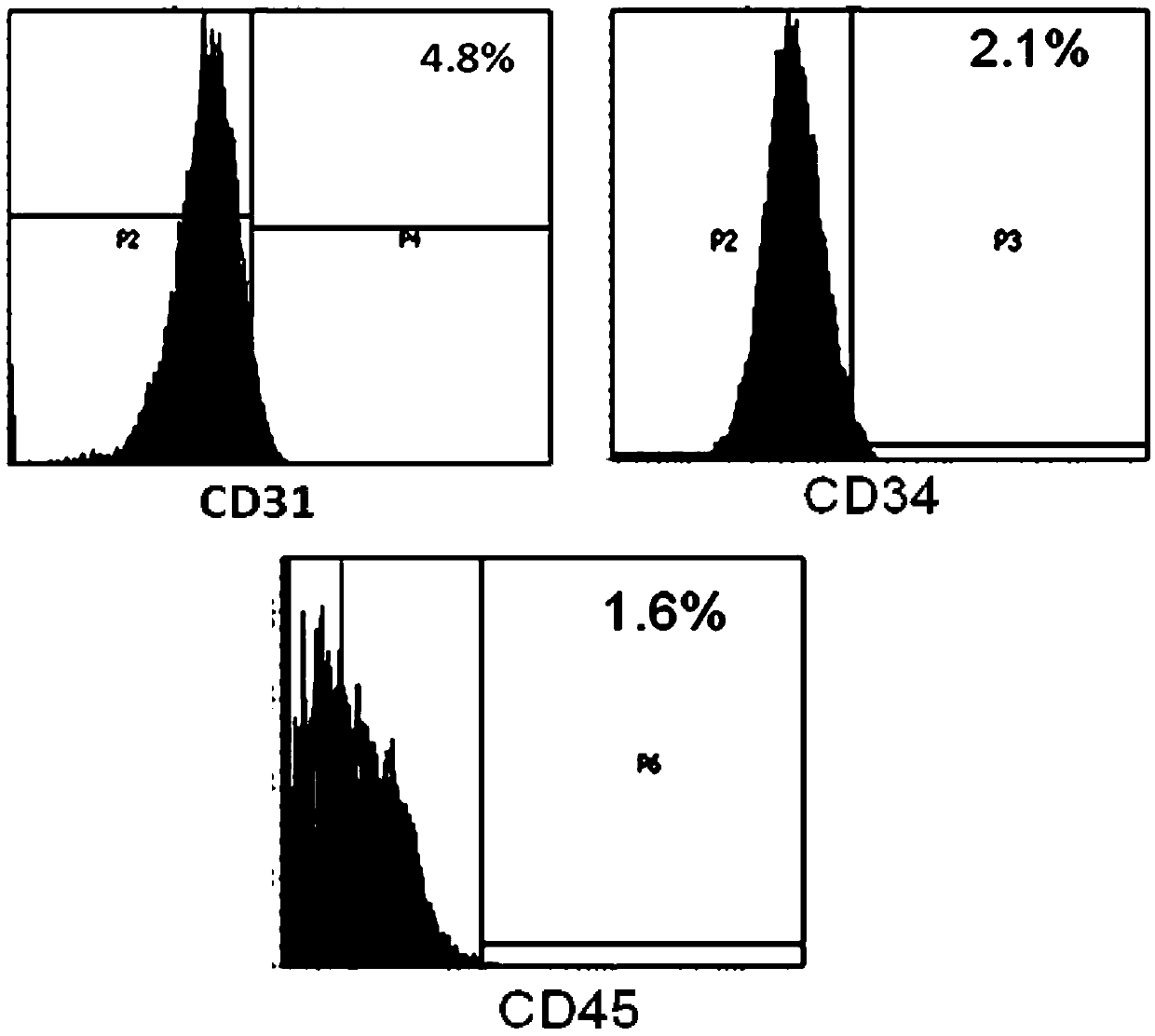
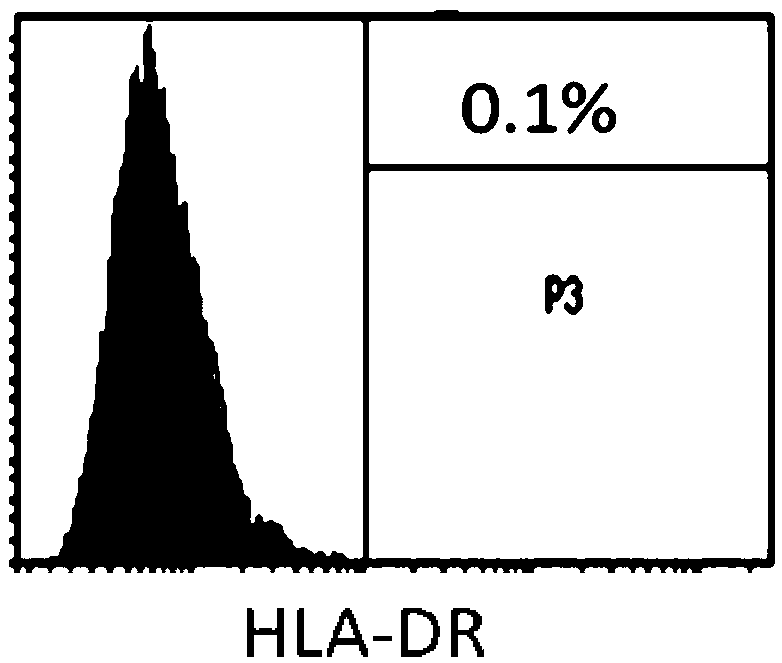
[0079] Use flow cytometry to detect the expression of CD29, CD44, CD90, CD105, CD126, CD31, CD45, CD34 and HLA-DR in the test product. The specific steps are as follows: After the cells (test product) are incubated with the corresponding antibodies respectively , Wash off excess antibodies, resuspend the cells in PBS buffer, and use BD’s LSR Fortessa instrument to detect the positive rate of each indicator.
[0080] The test results of mesenchymal stem cell markers (CD29, CD44, CD90, CD105 and CD126) are shown in figure 1 . For the test results of hematopoietic stem cell markers (CD31, CD45 and CD34), see figure 2 . For the test results of the markers related to transplant rejection (HLA-DR), see image 3 . The results showed that the test product expressed high expression of mesenchymal stem cell markers (both positive rates were above 97%), low e...
Embodiment 3
[0081] Example 3. The therapeutic effect of P3 cell preparation on rheumatoid arthritis
[0082] 1. Model preparation and administration
[0083] Test mice: 6-8 weeks, DBA / 1J mice, adapted to feed for one week (clean animal breeding room, free drinking water). Chicken type II collagen and Freund's adjuvant were used to sensitize mice to induce arthritis model. Divided into the following groups according to whether it is administered or not and the difference in administration:
[0084] Normal control group (5 mice): 0.1 mL of saline was used to subcutaneously inject each mouse's tail root. After 3 weeks, 0.1 mL of normal saline was injected subcutaneously into the base of each mouse's tail. From the day of the second injection, normal saline was injected into the tail vein once a week (100 μL per injection).
[0085] Cell therapy group (8 animals): use 0.1 mL of emulsified antigen adjuvant suspension (mixed with the same volume of 2 mg / mL chicken type II collagen solution and Freun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com