Virus sample preservation solution, nucleic acid extraction reagent and viral nucleic acid extraction method
A nucleic acid extraction reagent and viral nucleic acid technology, which is applied in the field of virus sample preservation solution, can solve the problems of reducing the concentration of viral nucleic acid, limited viral nucleic acid content, and the inability to increase the amount of samples, so as to optimize the process, increase the amount of sample input, and reduce pollution. or the effect of the risk of error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
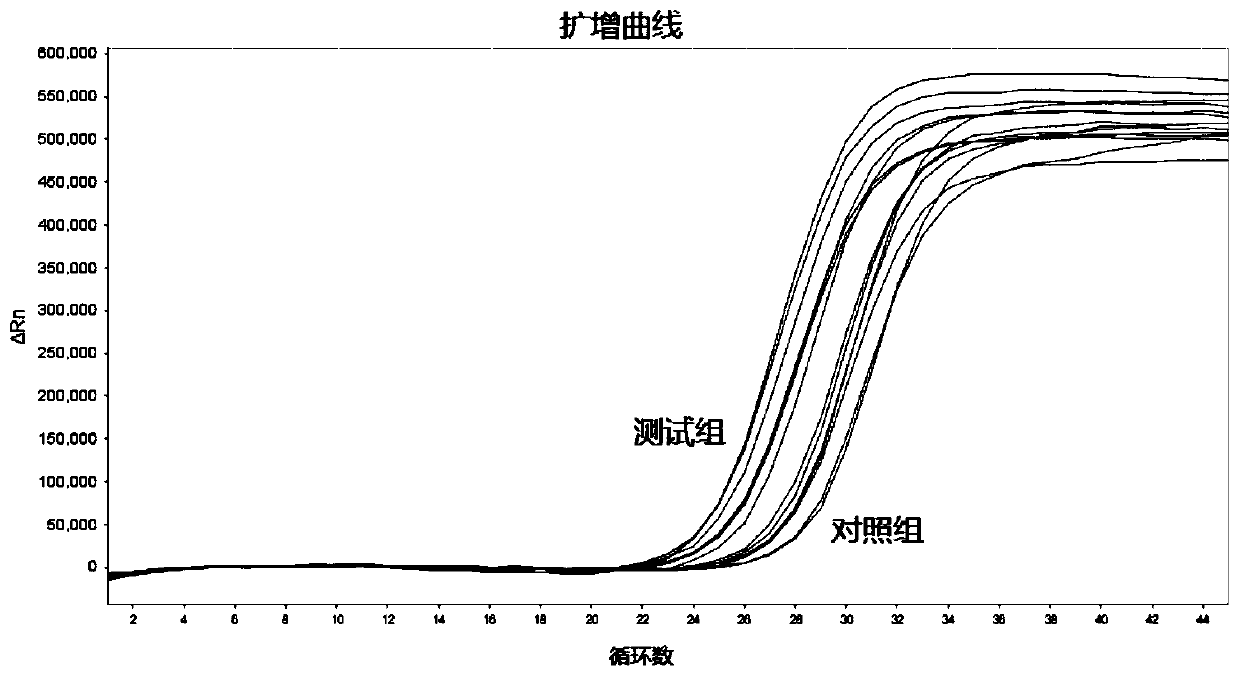
[0048] Embodiment 1 test group and control group sample preparation
[0049] Prepare enough virus sample preservation solution with cracking effect: guanidine thiocyanate (3.5mol / L), Tween-20 (volume percentage is 2%), polidocanol (volume percentage is 2%), ethanol (volume percentage Percentage is 30%), trisodium citrate (70mmol / L), citric acid (0.5mmol / L), dithiothreitol (mass volume percentage is 0.3g / 100mL);
[0050] Sample collection: 6 people. Each person collected throat swab samples 3 times and marked them. The 3 repeated samples were marked as -1, -2, -3 in turn.
[0051] Test group sample preparation: Add 19.5mL of freshly prepared virus sample preservation solution with lysing effect to the 50mL centrifuge tube, 10 5 PFU / mL MS2 pseudovirus 97.5μL (virus preservation solution volume: 10 5 MS2 volume of PFU / mL=200:1), mix thoroughly, and add 1.5mL of the mixture to the centrifuge tubes marked -2 and -3 respectively to obtain the test group samples.
[0052] Sample...
Embodiment 2
[0053] Example 2 Virus nucleic acid extraction and purification of control group sample
[0054] Viral nucleic acid extraction reagents and Shanghai Kangyu automatic nucleic acid purification instrument (model: KY-32A) were used for viral nucleic acid extraction and purification, and 6 samples numbered -1 in Example 1 were extracted to obtain corresponding viral nucleic acid samples.
[0055] experiment process:
[0056] Briefly centrifuge the pre-packaged reagent plate, tear off the heat-sealed aluminum film, add 200 μL of the control sample and 20 μL of proteinase K to the 6 wells of A1-F1 in the first row of the 96 deep-well plate, and place the reagent plate in the instrument compartment Put a new magnetic rod cover into the corresponding card slot at the fixed position inside.
[0057] In the virus nucleic acid extraction reagent provided: lysate (columns 1 and 7 of 96-well deep-well plate, 700 μL per well), including guanidine thiocyanate with a final concentration of 3...
Embodiment 3
[0067] Example 3 Test group sample virus nucleic acid extraction and purification
[0068] Viral nucleic acid extraction reagents and Shanghai Kangyu automatic nucleic acid purification instrument (model: KY-32A) were used for viral nucleic acid extraction and purification, and 6 samples numbered -2 in Example 1 were extracted to obtain corresponding viral nucleic acid samples.
[0069] experiment process:
[0070] Briefly centrifuge the pre-packaged reagent plate, tear off the heat-sealed aluminum film, discard the lysate in the 6 wells of A1-F1 in the first column of the 96 deep-well plate, and add 900 μL of test group samples, 20 μL of For proteinase K, place the reagent plate at a fixed position in the instrument compartment, and put a new magnetic rod cover into the corresponding card slot.
[0071] In the virus nucleic acid extraction reagent provided: proteinase K, the protein content is 15-30mg / mL. Cleaning solution I (columns 2 and 8 of 96-well deep-well plate, 90...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com