Impurity generated in production of indapamide as well as synthesis method and application
A kind of technology of indapamide and synthesis method, applied in the field of chemical substance preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
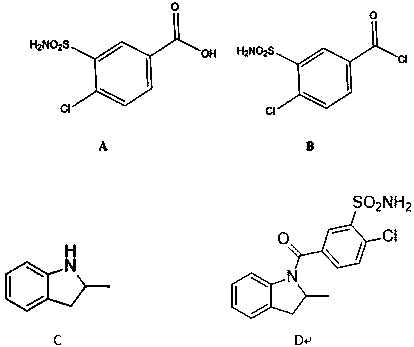
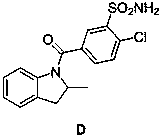
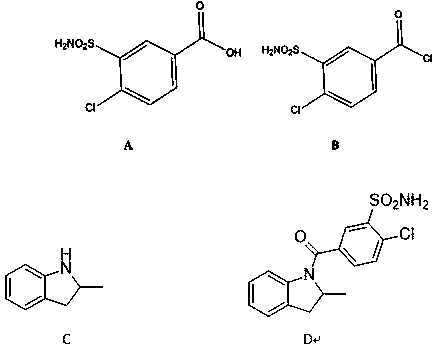
[0034] A synthetic method for impurity D produced in the production of indapamide, including the following steps:
[0035] 1) Add 10.00 g (0.042 mol) compound A, 25.4 g (0.0.21 mol) thionyl chloride, and 0.05 mL N,N- to a 250 ml three-neck flask equipped with a thermometer, a reflux tube, and mechanical stirring. Dimethylformamide, heat preservation at 80 ℃, heat preservation reaction for 4 hours, TLC monitors the reaction, after the reaction is complete, steam the thionyl chloride in the reaction solution, put the materials on the tray and put it in the oven, turn on the oven and heat up to 80°C, 8 hours. 10.3 g of compound B was obtained with a yield of 95.52%.
[0036] 2) Under the protection of nitrogen, add 5.00 g (0.37 mol) compound C, 9.50 g (0.93 mol) triethylamine, 100 ml dry tetrahydrofuran to a 250 ml three-neck flask equipped with a thermometer and mechanical stirring, and add 10 g ( 0.39 mol) Compound B was dissolved in 50 mL of tetrahydrofuran and added dropwise to ...
Embodiment 2
[0039] 1) Add 15.00 g (0.042 mol) compound A, 50.4 g (0.42 mol) thionyl chloride, and 0.05 mL of N,N-dimethyl into a 250 ml three-neck flask equipped with a thermometer, a reflux tube, and mechanical stirring. Methyl formamide, heat preservation at 80℃, heat preservation reaction for 4 hours, TLC monitors the reaction, after the reaction is over, distill the thionyl chloride in the reaction solution, put the materials on the tray and put it in the oven, turn on the oven and raise the temperature to 80℃ ,8 hours. 15.2 g of compound B was obtained with a yield of 95.52%.
[0040] 2) Under the protection of nitrogen, add 5.00 g (0.37mol) of compound C, 9.50 g (0.93 mol) of triethylamine, 100 ml of dry tetrahydrofuran to a 250 ml three-neck flask equipped with a thermometer and mechanical stirring, and add 10.5 g ( 0.39 mol) Compound B was dissolved in 50 mL of tetrahydrofuran and added dropwise to the reaction within 30 minutes. The reaction was carried out for 2 hours. The reactio...
Embodiment 3
[0044] Agilent 1200 high performance liquid chromatograph, octadecylsilane-bonded silica gel is used as filler, methanol-water-glacial acetic acid (volume ratio is 45:55:0.5) is used as mobile phase, 20 mg of indapamide reference substance is taken, Precisely weigh, put in a 100mL measuring flask, add 5mL methanol to dissolve, dilute to the mark with mobile phase, shake well, measure 5mL, add 1mol / L sodium hydroxide solution 2mL, put it in a water bath to heat for one hour, let cool , Adjust to neutral with 1 mol / L hydrochloric acid solution. Dilute to 50 mL with mobile phase. The injection volume is 100μl, the flow rate is 1~3ml / min, the detection wavelength is 246nm, and the column temperature is 30°C. Under the chromatographic conditions, indapamide peaked in about 12 minutes, and impurities peaked in about 24.8 minutes.
[0045] Take an appropriate amount of the impurity monomer and prepare a solution of 0.5 mg / ml with a diluent. The purity of the main peak is 98.87% calcul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com