Preparation method of magnetic Fe3O4 loaded dendrimer adsorbent
A dendrimer and adsorbent technology, applied in the field of preparation of magnetic Fe3O4-loaded dendrimer adsorbent, can solve the problems of uneven distribution of functional groups, low loading rate, adverse effects of adsorption, etc., and achieve good adsorption selectivity , the effect of high content of functional groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
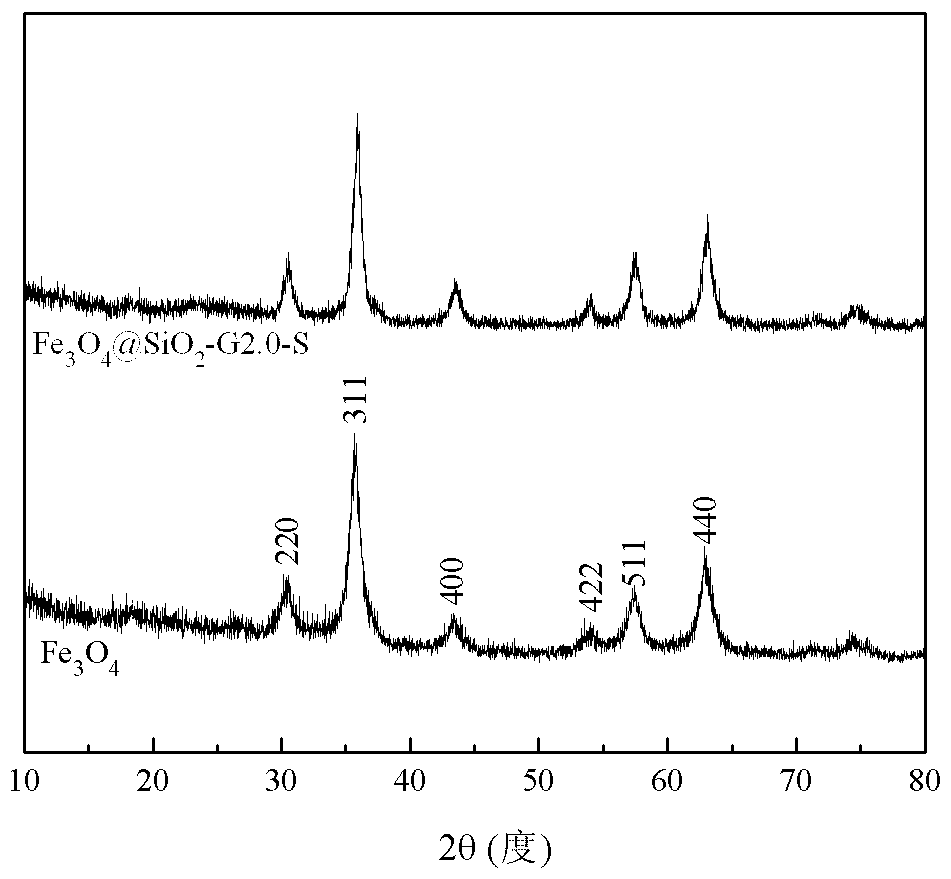
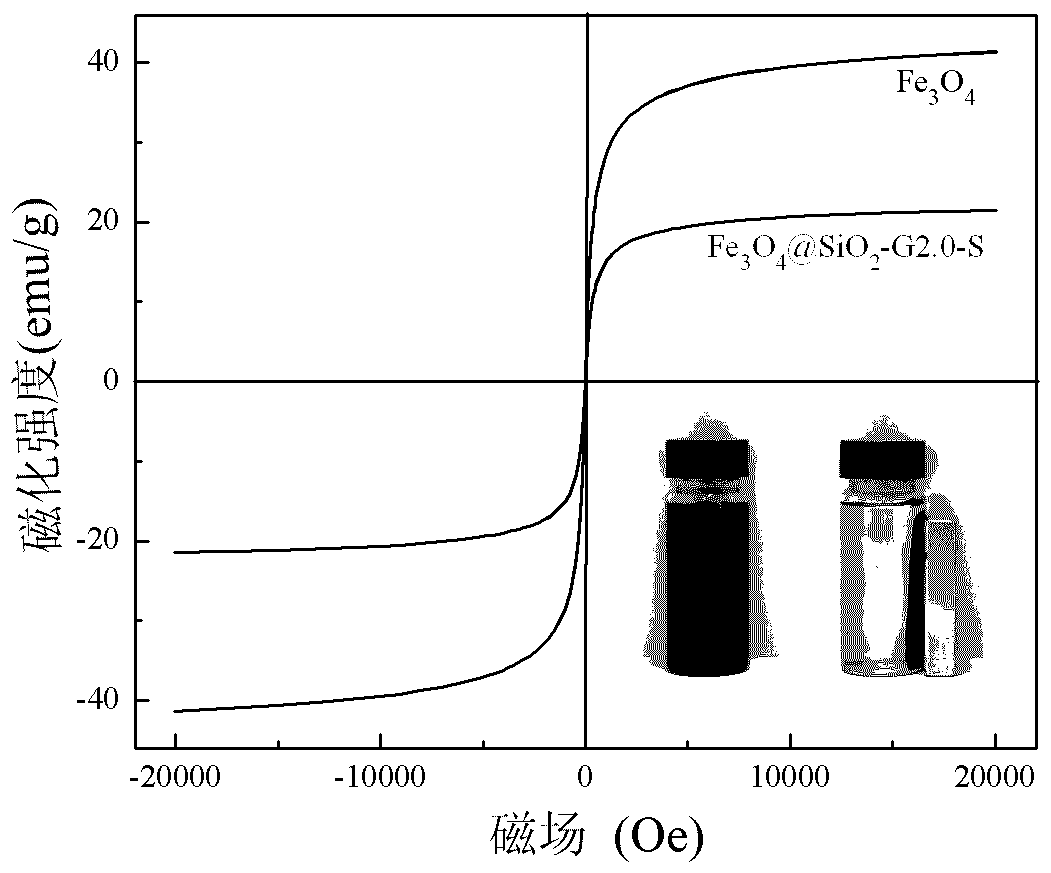
[0018] Under nitrogen protection, 5 g of Fe 3 o 4 @SiO 2 and 10 g of the 2.0th generation triethoxysilyl-based PAMAM dendrimer were added to a three-necked flask, and 100 ml of toluene was added as a solvent. The reaction mixture was ultrasonically dispersed and reacted for 18 hours at 80 ° C. After the reaction was completed, it was cooled to At room temperature, the product was separated by a magnet, and washed three times by adding 100 ml of absolute ethanol to a three-necked flask. Then add 100 milliliters of absolute ethanol and 11 grams of salicylaldehyde, heat and reflux to continue the reaction for 16 hours after ultrasonic dispersion is uniform, cool to room temperature after the reaction, separate the product by a magnet, reflux extraction with absolute ethanol for 12 hours, and dry to obtain Fe 3 o 4 @SiO 2 -G2.0-S.
Embodiment 2
[0020] Under nitrogen protection, 5 g of Fe 3 o 4 @SiO 2 and 15 g of the 2.0th generation triethoxysilyl-based PAMAM dendrimer were added to a three-necked flask, and 150 ml of toluene was added as a solvent. The reaction mixture was ultrasonically dispersed and reacted for 24 hours at 75 ° C. After the reaction was completed, it was cooled to At room temperature, the product was separated by a magnet, and washed three times by adding 100 ml of absolute ethanol to a three-necked flask. Then add 150 milliliters of absolute ethanol and 16 grams of salicylaldehyde, heat and reflux to continue the reaction for 20 hours after ultrasonic dispersion, and cool to room temperature after the end of the reaction. The product is separated by a magnet, extracted with absolute ethanol for 12 hours, and dried to obtain Fe 3 o 4 @SiO 2 -G2.0-S.
Embodiment 3
[0022] Under nitrogen protection, 5 g of Fe 3 o 4 @SiO 2 and 22 g of the 2.0th generation triethoxysilyl-based PAMAM dendrimer were added to a three-necked flask, and 150 ml of toluene was added as a solvent. The reaction mixture was ultrasonically dispersed and reacted for 36 hours at 70 ° C. After the reaction, it was cooled to At room temperature, the product was separated by a magnet, and washed three times by adding 100 ml of absolute ethanol to a three-necked flask. Then add 150 milliliters of absolute ethanol and 23 grams of salicylaldehyde, heat and reflux to continue the reaction for 24 hours after ultrasonic dispersion, and cool to room temperature after the reaction, separate the product by a magnet, reflux extraction with absolute ethanol for 12 hours, and dry to obtain Fe 3 o 4 @SiO 2 -G2.0-S.
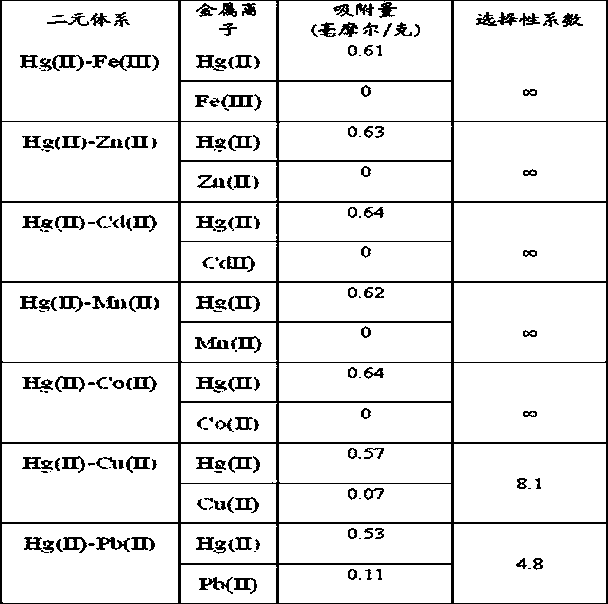
[0023] Performance Evaluation 1: Adsorption performance of the adsorbent prepared in Example 3 to metal ions
[0024] Add 20 ml of 0.001 mol / L Hg(II), Pb(II), Ni(II)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com