Dry heat sterilization indicating ink, preparation method and dry heat sterilization indicator
A dry heat sterilization and indication technology, applied in inks, instruments, household appliances, etc., can solve the problems of increasing the sterilization cost of medical kits, unqualified dry heat sterilization effect, misjudgment by sterilizers, etc. risk of judgment, avoid secondary sterilization, and enhance the effect of adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
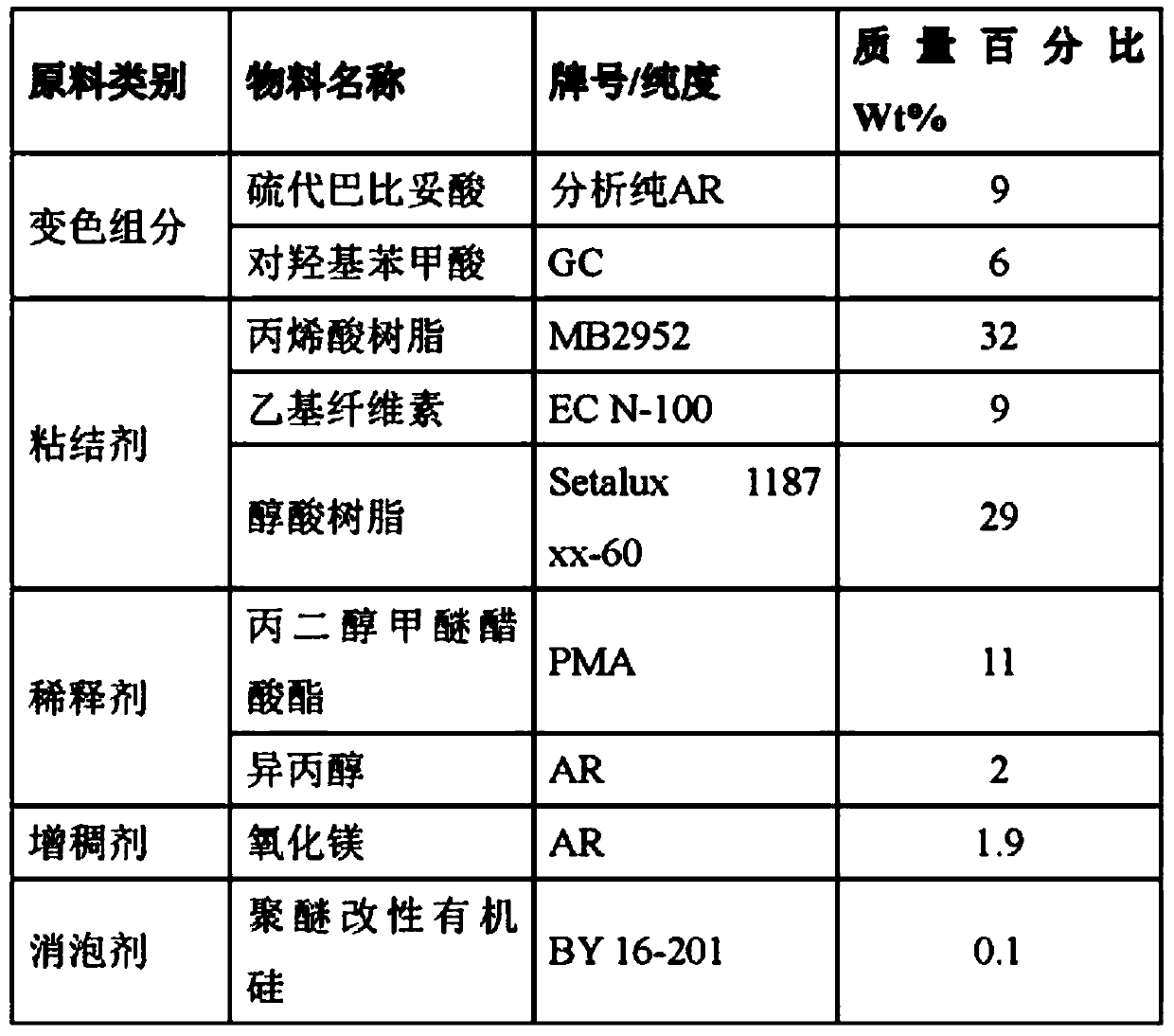
[0023] According to the material ratio in Table 1, dissolve acrylic resin, ethyl cellulose, and alkyd resin in the mixed solution of propylene glycol methyl ether acetate and isopropanol, continue stirring and dissolving for 12 hours to obtain a uniform transparent solution, and then add Thiobarbituric acid and p-hydroxybenzoic acid, stirred for 30 minutes, continued to add magnesium oxide, polyether modified silicone, stirred for 1 hour to obtain a uniform pre-mixed ink, and ground for 60 minutes with a pneumatic grinder to obtain the dry heat sterilization instruction ink. The ink is printed on masking tape by screen printing to obtain a dry heat sterilization indicator.
[0024] Table 1
[0025]
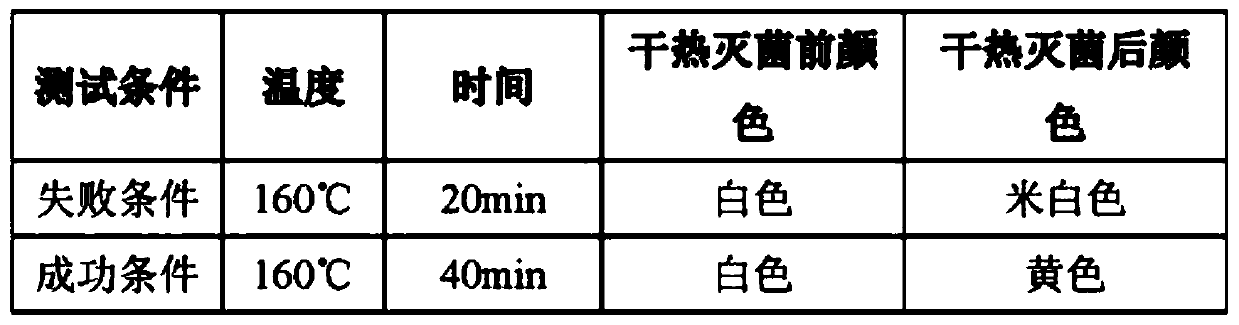
[0026] Dry the sample and place it in a dry heat sterilization resistance tester for testing. The test method refers to ISO11140-1. The test conditions and results are shown in Table 2:
[0027] Table 2
[0028]
[0029] The test results meet the requirements of GB18282.1...
Embodiment 2
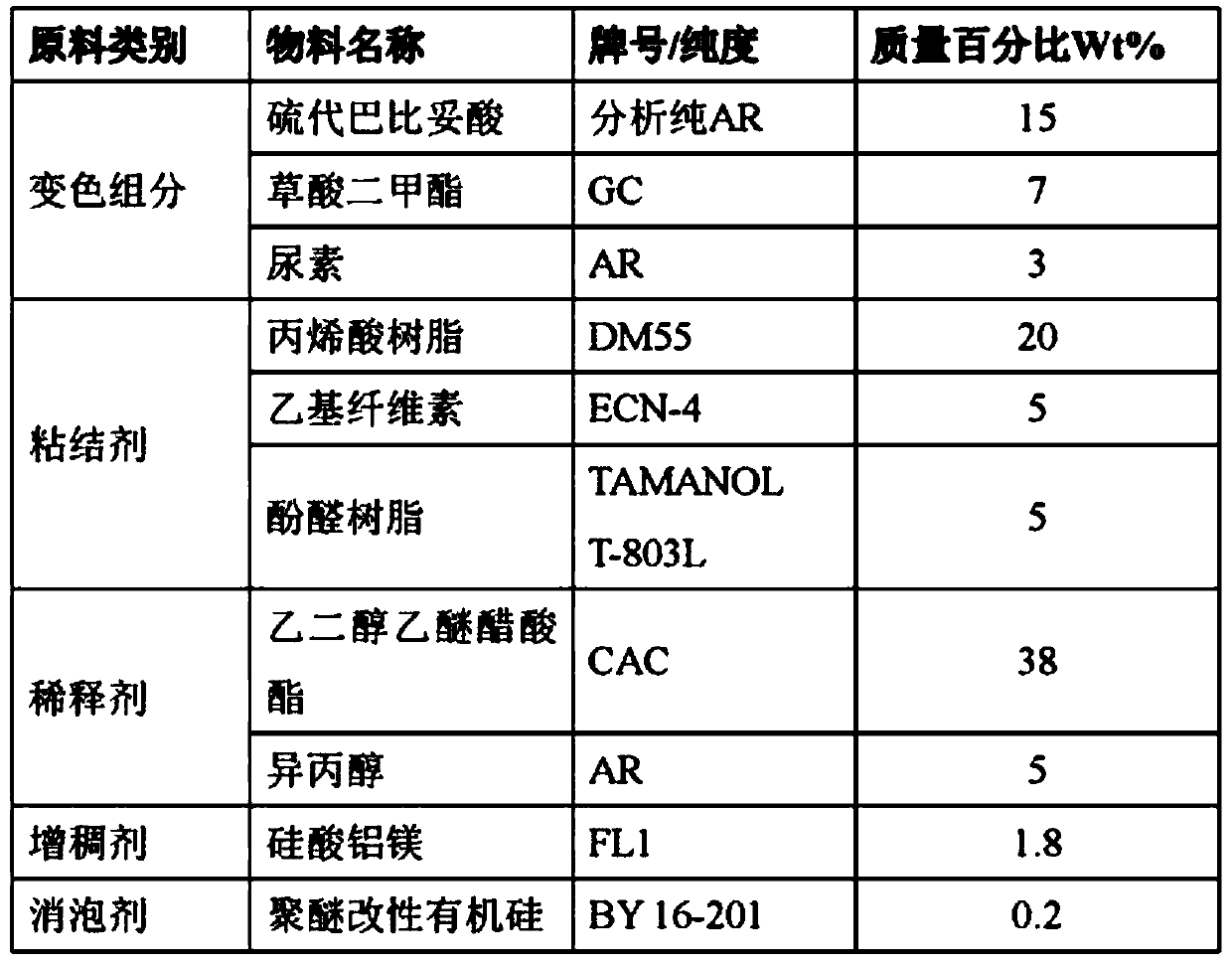
[0031] According to the material ratio in Table 3, dissolve acrylic resin, ethyl cellulose, and alkyd resin in the mixed solution of propylene glycol methyl ether acetate and isopropanol, and continue stirring and dissolving for 12 hours to obtain a uniform transparent solution, and then add Thiobarbituric acid, p-hydroxybenzoic acid, and urea, stirred for 30 minutes, continued to add magnesium oxide, polyether modified silicone, stirred for 1 hour to obtain a uniform pre-mixed ink, and ground for 75 minutes with a pneumatic grinder to obtain dry heat extinguishing ink. Bacteria indicator ink. The ink is printed on white cardboard by screen printing to obtain a dry heat sterilization indicator.
[0032] table 3
[0033]
[0034] Dry the sample and cut it into 2cm*10cm flakes, and place it in a dry heat sterilization resistance tester for testing. The test method refers to ISO11140-1. The test conditions and results are shown in Table 4:
[0035] Table 4
[0036]
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com