Fluoropyrrolopyrimidine compounds and their preparation methods and applications
A technology of fluoropyrrolopyrimidine and pyrrolopyrimidine, which is applied in the field of preparation of positron emission imaging agents, can solve problems such as unclear detailed etiology, and achieve the effect of benefiting radiation protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] This embodiment provides a 19 F-substituted fluoropyrrolopyrimidine compounds, which can be used for LRRK2 positron emission imaging, the preparation process and specific steps are as follows:
[0060]
[0061] Synthetic steps of compound 1:
[0062] A solution of 4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (1.96g, 7mmol) dissolved in tetrahydrofuran (50ml) was cooled to 0°C and washed with sodium hydride (60% in oil, 0.308g, 7.7mmol) was added in three portions. After stirring the reaction mixture at 0 °C for 1 h, 2-(trimethylsilyl)ethoxymethyl chloride (1.28 g, 7.6 mmol) was added dropwise, and the reaction mixture was warmed to room temperature and stirred for 4 h. The reaction was quenched with saturated aqueous sodium chloride (10m). The organic layer was dried over sodium sulfate, filtered and concentrated in vacuo. Silica gel chromatography (eluent, 10:1 petroleum ether / ethyl acetate), the product is a white solid, and the yield is about 55%.
[0063] Com...
Embodiment 2
[0087] This embodiment provides a 18 F-labeled fluoropyrrolopyrimidine compounds, the preparation method of which is as follows:
[0088]
[0089] The compound 3 prepared in Example 1 is used as a raw material to carry out the following steps:
[0090] [ 18 F]4 synthetic steps:
[0091] After 1 mg of compound 3 was dissolved in 200 μL DMSO, it was added to a dry [ 18 F] In the F-reaction bottle, press the cap, react at 150°C for 20 minutes to realize the labeling reaction, and obtain [ 18 F]4.
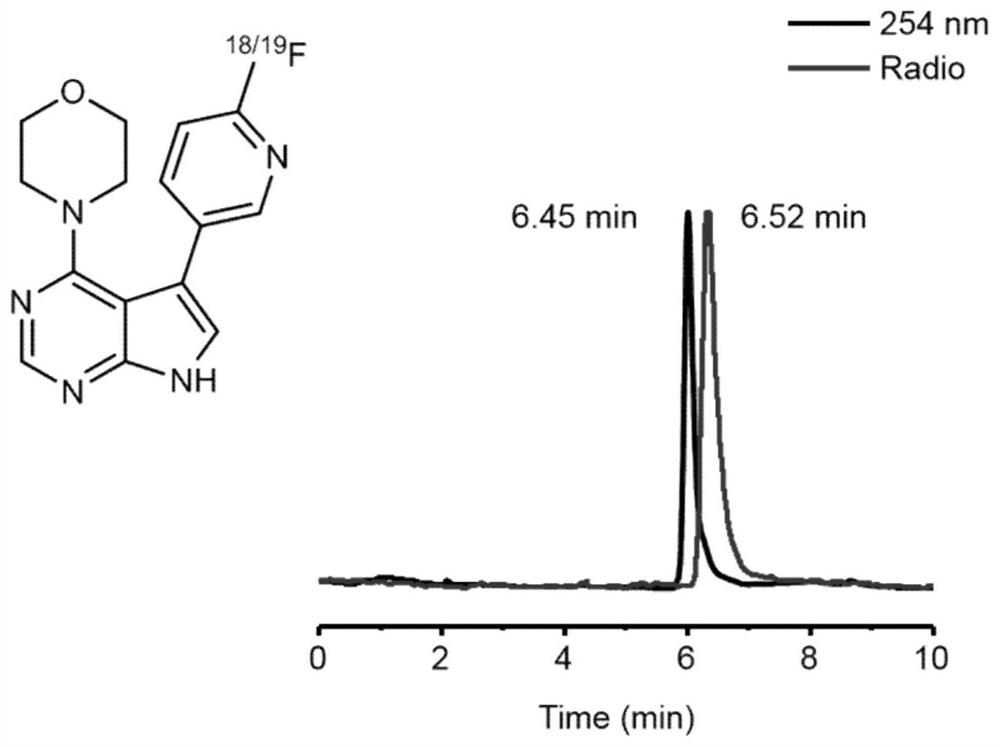
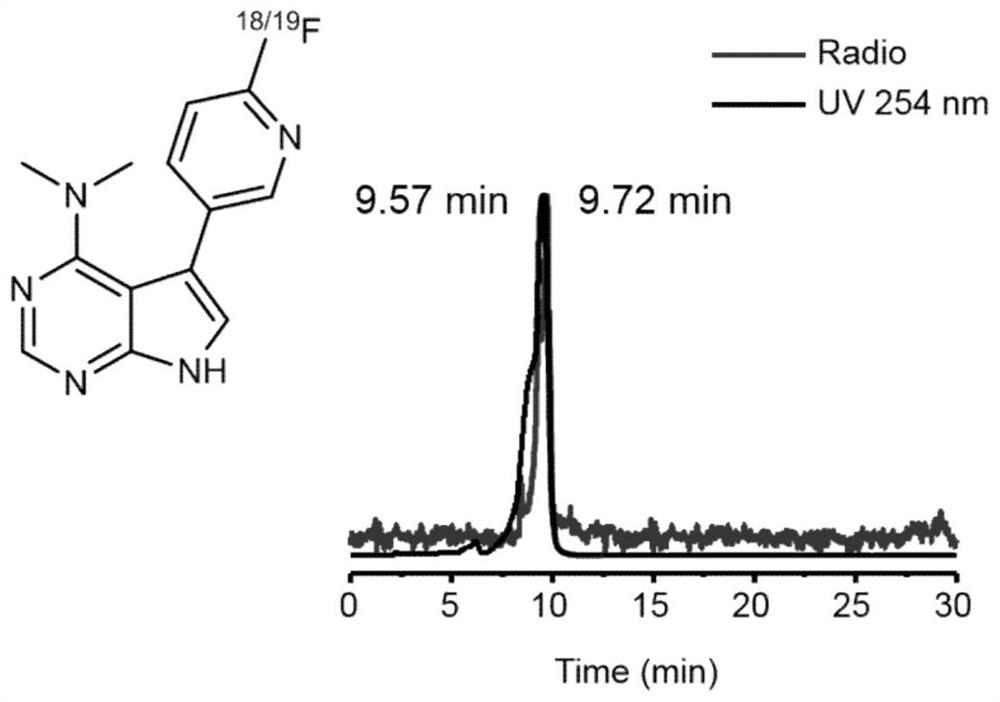
[0092] compound [ 18 F] 4 and compound 4 were co-injected into HPLC, suggesting that compound 3 was successfully labeled.
[0093] [ 18 Synthetic steps of F]5:
[0094] Will[ 18 F] After 4C18 column solid-phase extraction, rinse with 2 mL of dichloromethane, blow dry with nitrogen, add dichloromethane (200 μL) to dissolve, slowly add trifluoroacetic acid (200 μL) dropwise at 40 ° C and shake the cap for 5 min. The pH value of the reaction mixture was adjusted to neutral wi...
Embodiment 3
[0097] This embodiment provides a method for LRRK2 positron emission imaging 19 F-substituted pyrrolopyrimidine compounds, the preparation process and specific steps are as follows:
[0098]
[0099] Synthetic steps of compound 1:
[0100] A solution of 4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (1.96g, 7mmol) dissolved in tetrahydrofuran (50ml) was cooled to 0°C and washed with sodium hydride (60% in oil, 0.308g, 7.7mmol) was added in three portions. After stirring the reaction mixture at 0 °C for 1 h, 2-(trimethylsilyl)ethoxymethyl chloride (1.28 g, 7.6 mmol) was added dropwise, and the reaction mixture was warmed to room temperature and stirred for 4 h. The reaction was quenched with saturated aqueous sodium chloride (10m). The organic layer was dried over sodium sulfate, filtered and concentrated in vacuo. Silica gel chromatography (eluent, 10:1 petroleum ether / ethyl acetate), the product is a white solid, and the yield is about 55%.
[0101] Compound 1 NMR result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com