Method for preparing 3-acetamidofuran and derivatives thereof from marine waste biomass
A technology for acetamidofuran and waste biomass, applied in directions such as organic chemistry, can solve problems such as 3-acetamidofuran being rarely reported, and achieve the effects of widening the scope of effective utilization, improving economy, and being easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
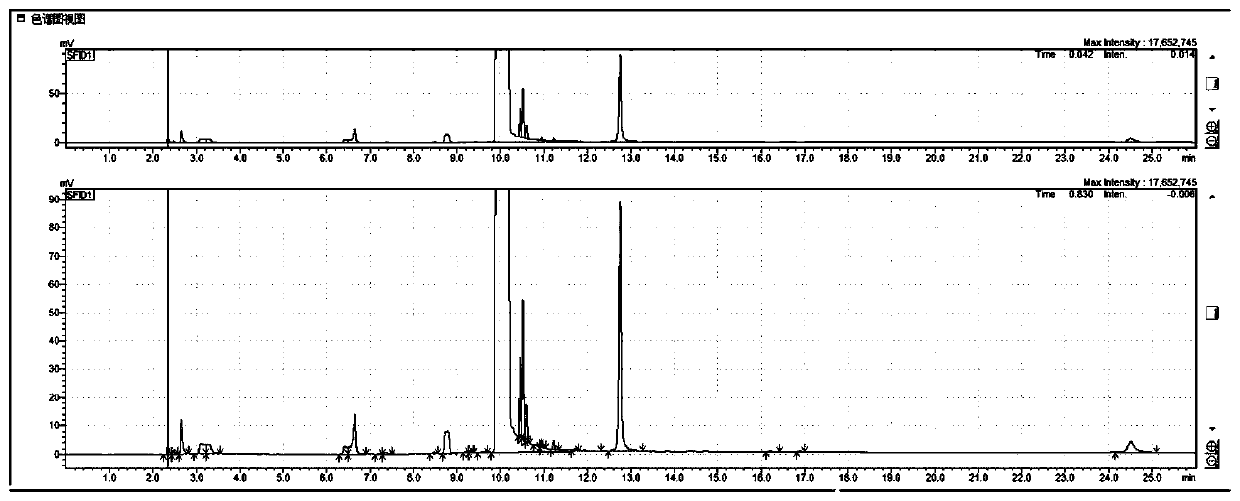
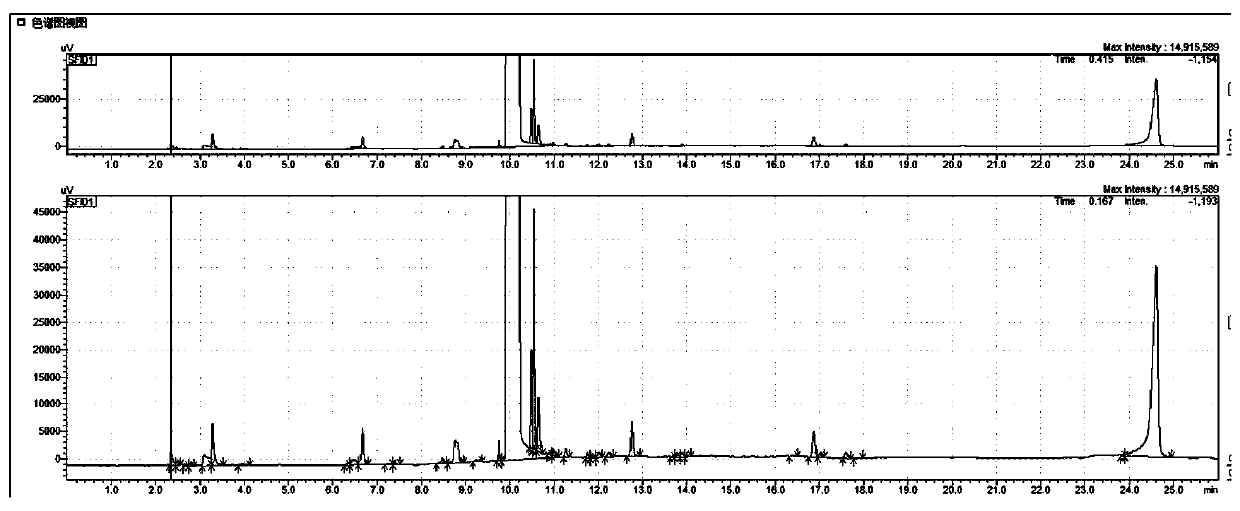
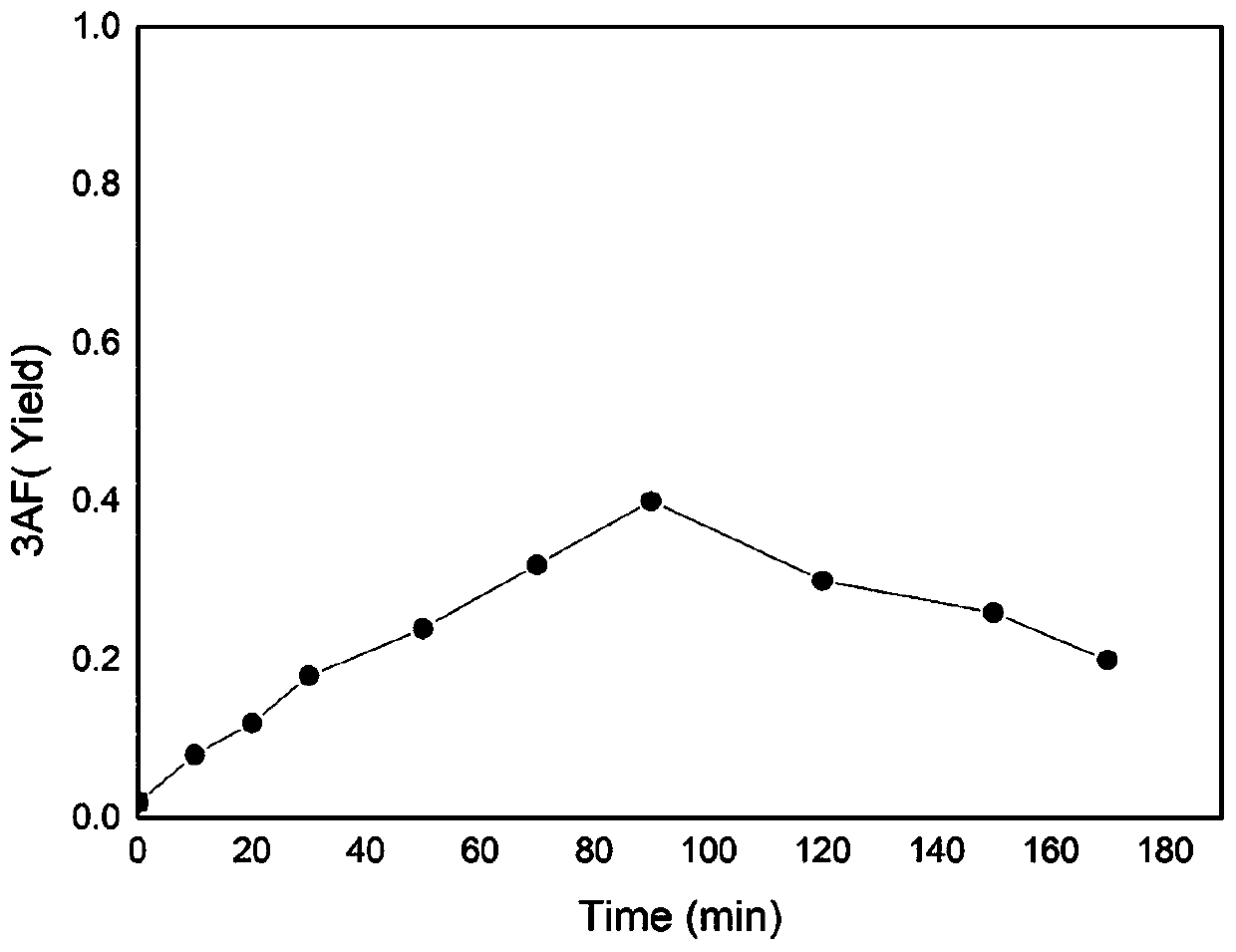
[0052] Take 0.22g of N-acetylglucosamine, 0.15g of barium hydroxide octahydrate, 0.12g of boric acid, and 0.10g of sodium chloride in a thick-walled pressure-resistant tube, add 10mL of N-methylpyrrolidone to dissolve, protect it with nitrogen, and keep it in a constant temperature oil bath Heat and stir at 180°C in the pot for 120 minutes. After the reaction was completed and cooled to room temperature, 0.5 mL of the filtrate was taken, filtered through a nylon microporous membrane, and the content of 3-acetylaminofuran was detected by gas phase, and the yield of the obtained 3-acetylaminofuran was 58%.
Embodiment 2
[0058] Take 0.42g of N-acetylglucosamine, 0.05g of lithium hydroxide monohydrate, 0.15g of fluorophenylboronic acid, and 0.22g of calcium chloride in a round bottom flask, protect it with nitrogen, and add 10mL of N,N-dimethylformamide , heated at 190°C in a constant temperature oil bath with stirring and reflux for 100 minutes. After the reaction was completed and cooled to room temperature, 0.5 mL of the filtrate was taken, filtered through a nylon microporous membrane, and the content of 3-acetylaminofuran was detected by gas phase. Under the current assay conditions, the yield of 3-acetylaminofuran obtained was 40%.
Embodiment 3
[0060] Take 0.8g of chitin, 0.3g of potassium carbonate, 0.35g of chlorophenylboronic acid, and 0.12g of choline chloride in a thick-walled pressure-resistant tube, protect it with nitrogen, add 10mL of N,N-dimethylacetamide to dissolve, and keep the temperature Heat and stir at 200°C in an oil bath for 150 minutes. After the reaction was completed and cooled to room temperature, 0.5 mL of the filtrate was taken, filtered through a nylon microporous membrane, and the content of 3-acetylaminofuran was detected by gas phase. Under the current assay conditions, the yield of 3-acetylaminofuran obtained was 12%. When chitin is used as a reaction raw material, because it needs to be depolymerized and hydrolyzed into N-acetylglucosamine first, the yield of 3-acetylaminofuran is lower than that of using N-acetylglucosamine as a raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com