Modified conjugated diene-based polymer and method for preparing same
一种改性剂、化合物的技术,应用在所述聚合物的制备领域,能够解决轮胎物理性能的改善效果不显著、低末端改性比等问题,达到改善燃料消耗率、高改性比、亲和性提高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The production method according to one embodiment of the present invention is characterized in comprising: performing a first reaction between a compound represented by the following formula 4 and a compound represented by the following formula 5 to prepare a compound represented by the following formula 6 (step a ); and carry out the second reaction (step b) between the compound represented by the following formula 6 and the compound represented by the following formula 7:
[0090] [Formula 4]
[0091] h 2 N-R 4 -NH 2
[0092] [Formula 5]
[0093]
[0094] [Formula 6]
[0095]
[0096] [Formula 7]
[0097] R 9 R 10 R 11 SiCl
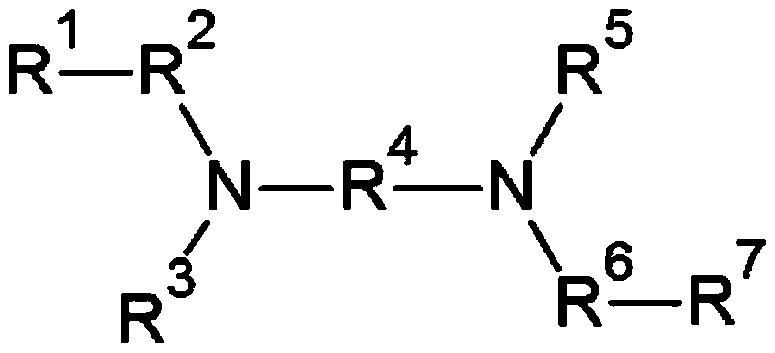
[0098] [Formula 1]
[0099]
[0100] In formula 1,
[0101] R 1 Yes - COOR 8 ,
[0102] R 2 and R 6 each independently being a divalent hydrocarbon group of 2 to 20 carbon atoms,
[0103] R 3 and R 5 Each independently is -SiR 9 R 10 R 11 ,
[0104] R 4 It is a divalent hydrocarbon group of 1 to 20 carbon atoms, ...
preparation Embodiment 1
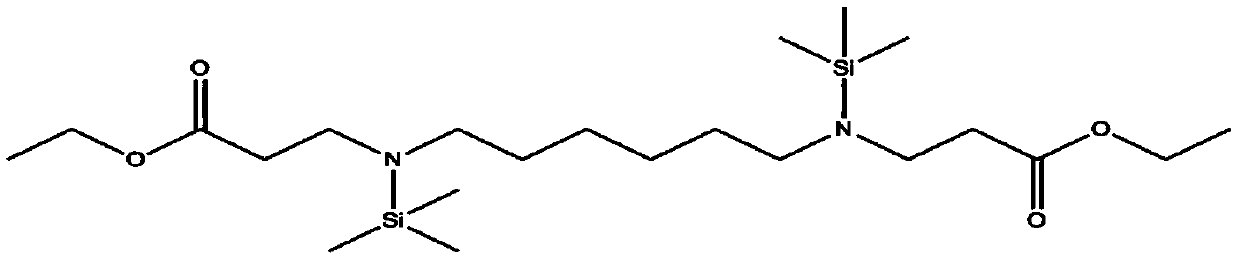
[0257] 1) Preparation of compounds represented by formula 2-1
[0258] To a 1 L round bottom flask, 46.5 g (400 mmol) of hexamethylenediamine and 88.2 g (880 mmol, 2.2 equivalents) of ethyl acrylate were slowly added. Then, a reaction was performed by stirring at room temperature for 12 hours. After checking the complete consumption of hexamethylenediamine, the reaction was terminated and the solvent was removed under reduced pressure. After that, the resulting product was filtered through a celite pad using 500 ml of n-hexane and concentrated to obtain 120.2 g (95% yield) of a compound represented by the following formula 2-1. The thus obtained compound represented by formula 2-1 1 H NMR spectral data are as follows.
[0259] [Formula 2-1]
[0260]
[0261] 1 H-NMR (500MHz, CDCl 3 )4.14(4H,q),2.86(4H,t),2.67(2H,td,J=7.0Hz,3.5Hz),2.59(2H,t),2.50(2H,t),1.50(4H,m) ,1.33(4H,m),1.25(3H,t)
[0262] 2) Preparation of compounds represented by formula 2
[0263] Into a 2L ...
preparation Embodiment 2
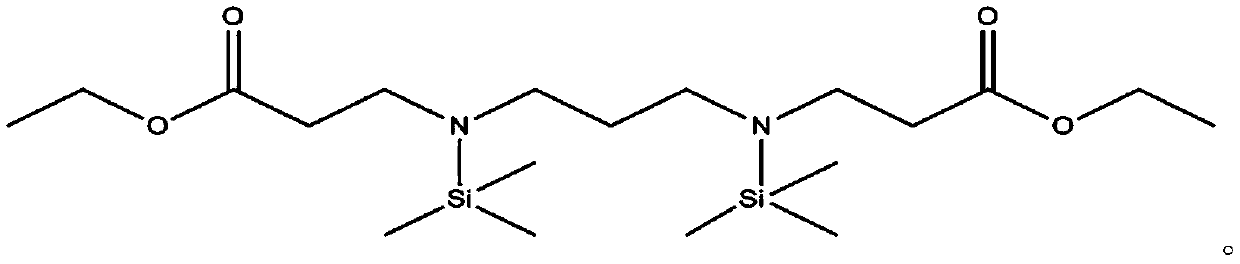
[0268] 1) Preparation of compounds represented by formula 3-1
[0269] To a 1 L round bottom flask, 29.6 g (400 mmol) of 1,3-diaminopropane and 88.2 g (880 mmol, 2.2 equivalents) of ethyl acrylate were slowly added. Then, a reaction was performed by stirring at room temperature for 12 hours. After checking the complete consumption of 1,3-diaminopropane, the reaction was terminated and the solvent was removed under reduced pressure. After that, the resulting product was filtered through a celite pad using 500 ml of n-hexane and concentrated to obtain 104.2 g (95% yield) of a compound represented by the following Formula 3-1. The thus obtained compound represented by formula 3-1 1 H NMR spectral data are as follows.
[0270] [Formula 3-1]
[0271]
[0272] 1 H-NMR (500MHz, CDCl 3 )4.14(4H,q),2.86(4H,t),2.59(4H,t),2.50(4H,t),1.50(2H,m),1.25(6H,t)
[0273] 2) Preparation of compounds represented by formula 3
[0274] Into a 2L round bottom flask was added 120.2g (380mmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com