Modifier, modified conjugated diene-based polymer comprising same, and method for preparing polymer
A conjugated diene and polymer technology, applied in the field of modified conjugated diene polymers, can solve the problems of insufficient effect, poor dispersion performance, low affinity, etc. The effect of excellent tensile strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0136] Preparation method of modified conjugated diene polymer
[0137] In addition, the present invention provides a preparation method of the modified conjugated diene polymer.
[0138] A method for preparing a modified conjugated diene polymer according to one embodiment of the present invention is characterized by comprising: making a conjugated diene monomer, or making an aromatic vinyl compound polymerizing a monomer and a conjugated diene-based monomer to prepare an organometallic-bonded living polymer (step S1); and reacting the living polymer with a modifier of a compound represented by the following formula 1 (step S2) .
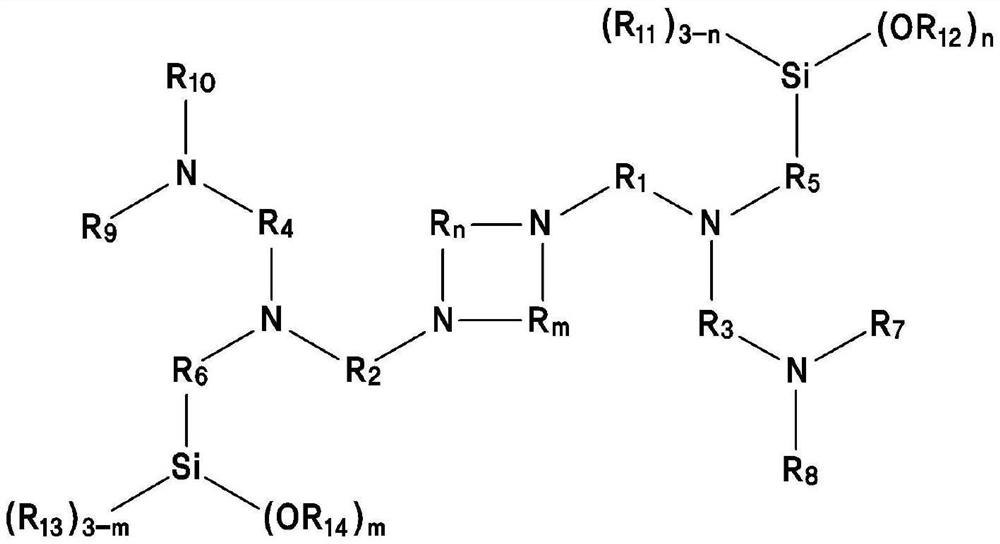
[0139] [Formula 1]
[0140]
[0141] The definition of each substituent of Formula 1 is the same as above.
[0142] Step 1 is a step of preparing a living polymer incorporating an organometallic compound, and can be obtained by making a conjugated diene-based monomer, or an aromatic vinyl-based monomer and a conjugated diolefin in a hydroca...
preparation Embodiment 1
[0183] (1)N 1 ,N 1 -Dimethyl-N 3 Preparation of -(3-(trimethoxysilyl)propyl)propane-1,3-diamine
[0184] Add 1.22 g (10 mmol) of 3-chloro-N,N-dimethylpropan-1-amine and 3.59 g (20 mmol) of 3-(trimethoxysilyl) to a 1 L round bottom flask connected to a schlenk line yl) propan-1-amine, and the water was completely removed under reduced pressure. Then, under an argon atmosphere, 500 ml of pyridine was added thereto and reacted for 10 hours while refluxing to prepare intermediate N 1 ,N 1 -Dimethyl-N 3 -(3-(Trimethoxysilyl)propyl)propane-1,3-diamine (80% yield). After purification, observe 1 H NMR spectrum, and confirm the synthesis.
[0185] 1 H NMR (CDCl 3 , 500MHz): δ3.55(s, 9H), 2.53-2.55(m, 4H), 2.36-2.40(m, 2H), 2.15(s, 6H), 1.53-1.60(m, 2H), 1.50(s , 1H), 1.33-1.39 (m, 2H), 0.50-0.56 (m, 2H).
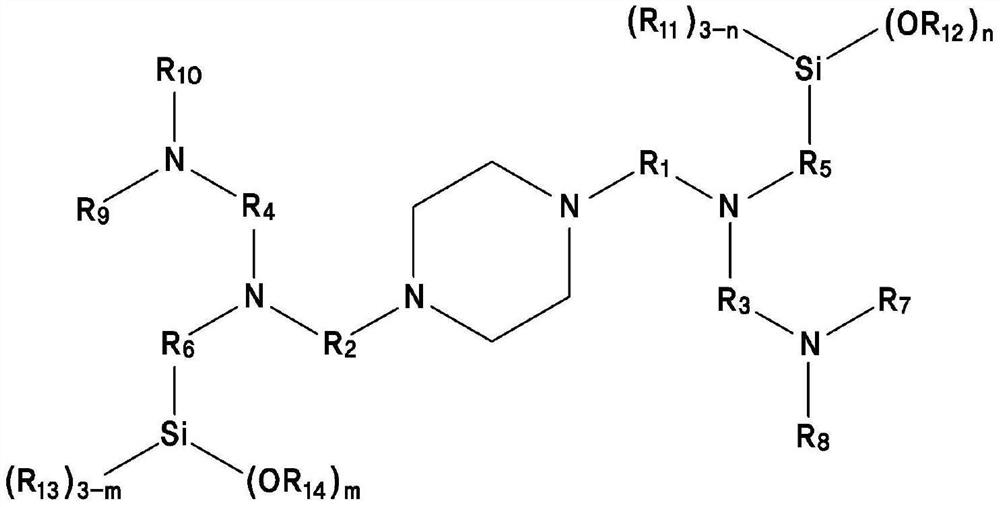
[0186] (2)N 1 ,N 1 -(piperazine-1,4-diylbis(propane-3,1-diyl))bis(N 3 ,N 3 -Dimethyl-N 1 Preparation of -(3-(trimethoxysilyl)propyl)propane-1,3-diamine)
[0187] To...
preparation Embodiment 2
[0193] (1)N 1 -(3-(Dimethoxy(methyl)silyl)propyl)-N 3 ,N 3 - Preparation of dimethylpropane-1,3-diamine
[0194] Add 1.22 g (10 mmol) of 3-chloro-N,N-dimethylpropan-1-amine and 3.27 g (20 mmol) of 3-(dimethoxy( methyl)silyl)propan-1-amine, and the water was completely removed under reduced pressure. Then, under an argon atmosphere, 500 ml of pyridine was added thereto and reacted for 10 hours while refluxing to prepare intermediate N 1 -(3-(Dimethoxy(methyl)silyl)propyl)-N 3 ,N 3 -Dimethylpropane-1,3-diamine (80% yield). After purification, observe 1 H NMR spectrum, and confirm the synthesis.
[0195] 1 H NMR (CDCl 3 ,500MHz): δ3.55(s,9H),2.53-2.55(m,4H),2.36-2.40(m,2H),2.15(s,6H),1.53-1.60(m,2H),1.50(s ,1H), 1.33-1.39(m,2H), 0.59-0.62(m,2H), 0.15(s,3H).
[0196] (2)N 1 , N 1 -(piperazine-1,4-diylbis(propane-3,1-diyl))bis(N 1 -(3-(Dimethoxy(methyl)silyl)propyl)-N 3 ,N 3 - Preparation of dimethylpropane-1,3-diamine)
[0197] To a 1 L round bottom flask connect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com