Novel compound, and modified conjugated diene-based polymer containing functional group derived from compound
A compound and selected technology, applied in the fields of organic chemistry, rolling resistance optimization, road transportation emission reduction, etc., can solve the problems of low end modification ratio, insignificant improvement effect of tire physical properties, etc., and achieve excellent affinity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The method for preparing a compound according to one embodiment of the present invention is characterized in that it includes: performing a first reaction of a compound represented by the following formula 2 with a compound represented by the following formula 3 to prepare a compound represented by the following formula 4 (step a ); and carrying out the second reaction (step b) of the compound represented by the formula 4 with the compound represented by the following formula 5:
[0085] [Formula 2]
[0086]
[0087] [Formula 3]
[0088]
[0089] [Formula 4]
[0090]
[0091] [Formula 5]
[0092]
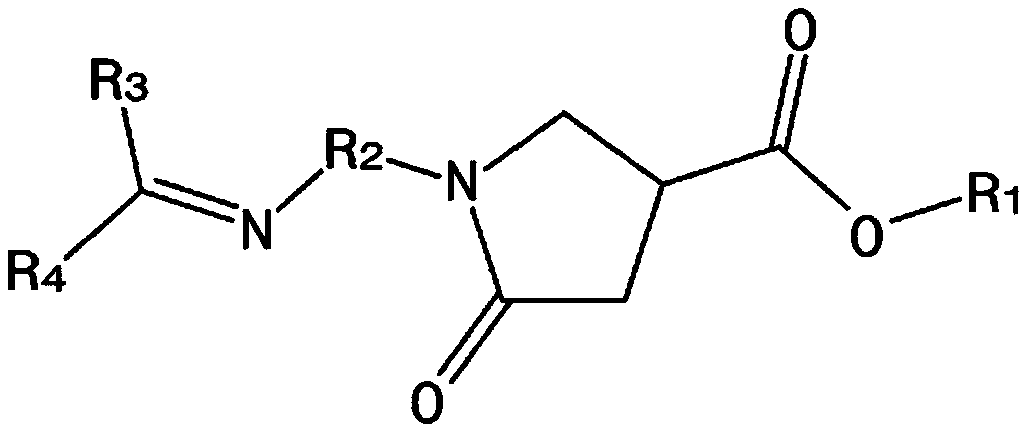
[0093] [Formula 1]
[0094]
[0095] In Formula 1 to Formula 5, R 1 to R 4 Same as described above.
[0096]The first reaction in step a is a step for preparing the compound represented by Formula 4, and may be performed by reacting the compound represented by Formula 2 with the compound represented by Formula 3.
[0097] In addition, the second reaction...
preparation Embodiment 1
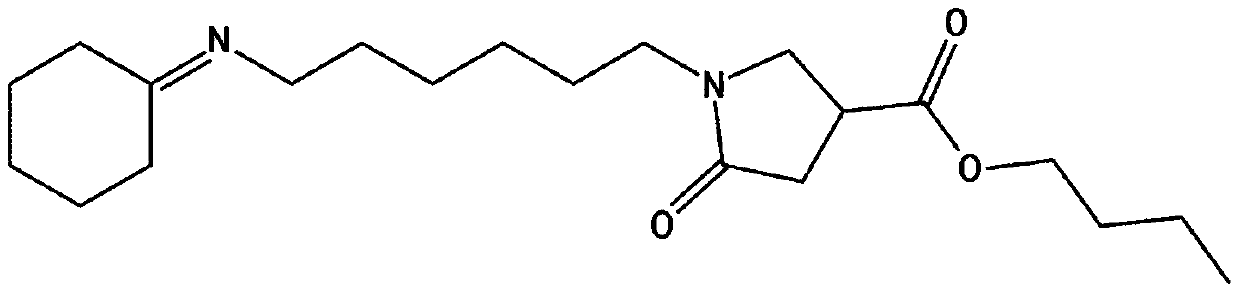
[0220] Preparation Example 1: Preparation of butyl 1-(6-(cyclohexylideneamino)hexyl)-5-oxopyrrolidine-3-carboxylate (i)
[0221] 1) Preparation of 6-(cyclohexylideneamino)hexane-1-amine
[0222] In the round bottom flask of 250ml, add the 1,6-hexamethylenediamine of 9.0g (77.45mmol), and add the cyclohexanone of 24.08ml (232.34mmol), then, use Dean-Stark apparatus to remove Water is produced and the temperature is raised to 150°C. Then, the reaction was performed for 12 hours, the reaction was terminated, distillation was performed to remove the remaining cyclohexanone, and 14.64 g (yield 96.3%) of 6-(cyclohexylideneamino)hexan-1-amine was obtained. 6-(Cyclohexylideneamino)hexan-1-amine 1 H NMR spectral data are as follows.
[0223] 1 H-NMR (500MHz, CDCl 3 )δ3.26-3.24(t,2H),2.67-2.64(t,2H),2.25-2.23(t,4H),1.70-1.68(m,2H),1.61-1.56(m,6H),1.43- 1.39(t,2H),1.33-1.31(m,4H),1.11-0.95(br,2H).
[0224] 2) Preparation of butyl 1-(6-(cyclohexylideneamino)hexyl)-5-oxopyrrolidine-...
preparation Embodiment 2
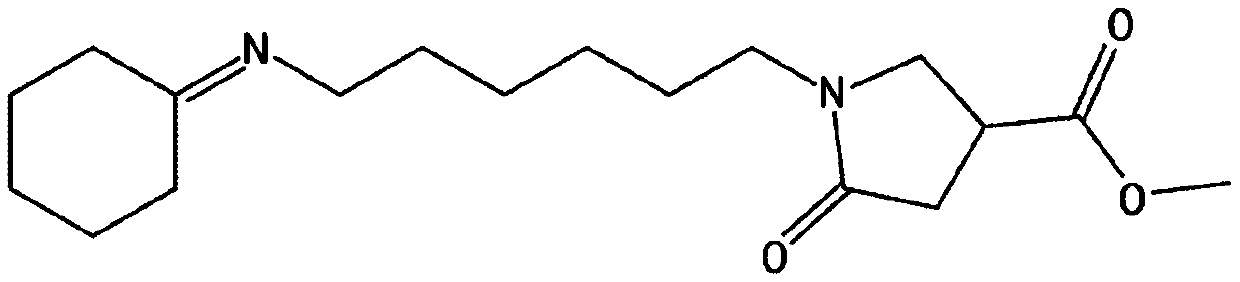
[0228] Preparation Example 2: Preparation of 1-(6-(cyclohexylideneamino)hexyl)-5-oxopyrrolidine-3-carboxylic acid methyl ester (ii)
[0229] 1) Preparation of 6-(cyclohexylideneamino)hexane-1-amine
[0230] In the round bottom flask of 250ml, add the 1,6-hexamethylenediamine of 9.0g (77.45mmol), and add the cyclohexanone of 24.08ml (232.34mmol), then, use Dean-Stark apparatus to remove Water is produced and the temperature is raised to 150°C. Then, the reaction was performed for 12 hours, the reaction was terminated, distillation was performed to remove remaining cyclohexanone, and 14.64 g (yield: 96.3%) of 6-(cyclohexylideneamino)hexan-1-amine was obtained. The preparation process was repeated twice to give a total of 29.28 g. 6-(Cyclohexylideneamino)hexan-1-amine 1 H NMR spectral data are as follows.
[0231] 1 H-NMR (500MHz, CDCl 3 )δ3.26-3.24(t,2H),2.67-2.64(t,2H),2.25-2.23(t,4H),1.70-1.68(m,2H),1.61-1.56(m,6H),1.43- 1.39(t,2H),1.33-1.31(m,4H),1.11-0.95(br,2H).
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com