A method for large-scale purification of gram-level mechanically functional proteins
A large-scale, mechanical technology, applied in the field of bioengineering, can solve the problems of difficult control of reversible phase transition conditions, and achieve the effect of easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Sample pretreatment
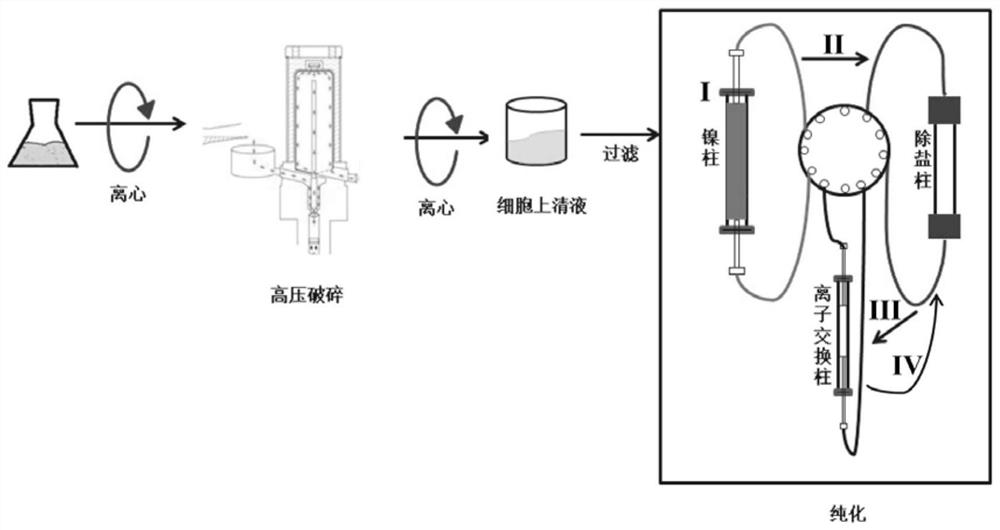
[0055] Put 2L of Escherichia coli fermentation broth containing SRT protein into a centrifuge, centrifuge in batches, 8000rpm for 20min, collect the precipitate, wash with equilibration buffer A and centrifuge in the same way, repeat three times. A total of 100g of bacterial cells were collected, and then 400ml of equilibrium buffer A was added. After crushing by a high-pressure crusher, centrifuge at 8000rpm for 20min. Dilute to 200mL to finally obtain 200mL of pretreated protein samples.
[0056] 2. Nickel column purification of SRT protein
[0057] Purify the pretreated protein obtained in step 1 through a nickel affinity chromatography column, the column used is HisTrap6XFF 5ml, and the specific purification steps are:
[0058] 2.1 Buffer preparation
[0059] (1) Equilibration buffer A:
[0060]
[0061] Add HCl to adjust pH=8.0
[0062] (2) Elution buffer A:
[0063] Equilibrium buffer A was used to prepare a buffer with a final co...
Embodiment 2
[0119] 1. Sample pretreatment
[0120] Put 20L E. coli fermentation broth containing recombinant Resilin protein into a continuous flow centrifuge, collect the precipitate continuously, wash with equilibration buffer A and centrifuge in the same way, repeat three times. A total of 1 kg of bacteria was collected, and then 5L of equilibrium buffer A was added, crushed by a high-pressure crusher, centrifuged at 8000rpm for 20min, and the obtained supernatant was filtered with a 0.45μm filter membrane to remove bacteria while removing macromolecules, and finally obtained 5L of pre- Process protein samples.
[0121] 2. Purification of Resilin protein by large-volume nickel column
[0122] Purify the pretreated protein obtained in step 1 through a large-volume nickel affinity chromatography column, and the filler used is NiSepharose TM 6Fast Flow high-flow nickel affinity chromatography packing material, the total nickel ion binding capacity of the packing material is not less tha...
Embodiment 3、4
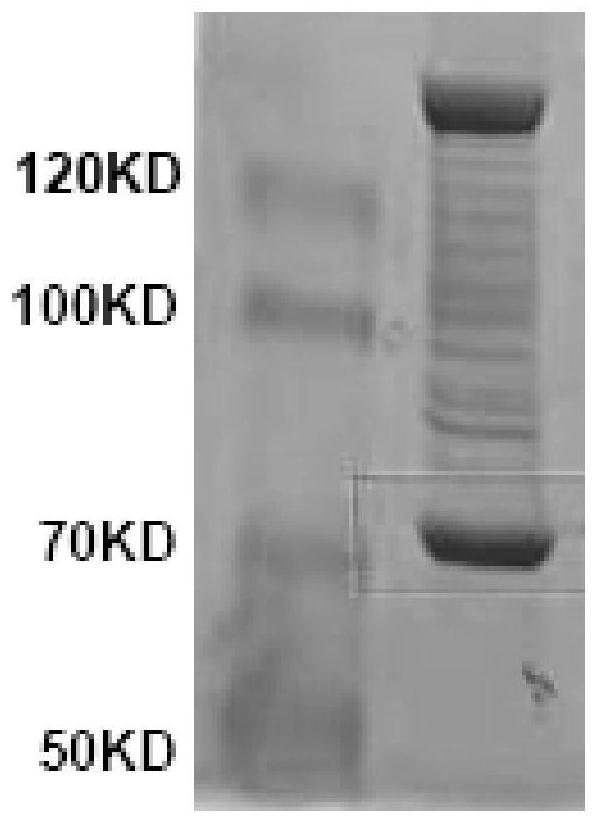
[0182] Similar to the operation method in Example 2, the difference is that the protein is replaced by spidroin and elastin. see results Figure 4-5 .
[0183] The results show, Figure 4 The spidroin protein purity in it reaches 90%, which meets the requirements. Figure 5 The purity of the individual ELPs in is very high, and its purity reaches 99% after analysis, which shows that the expression effect and purification effect of this tag are excellent, and it is extremely effective as a fusion expression tag.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com