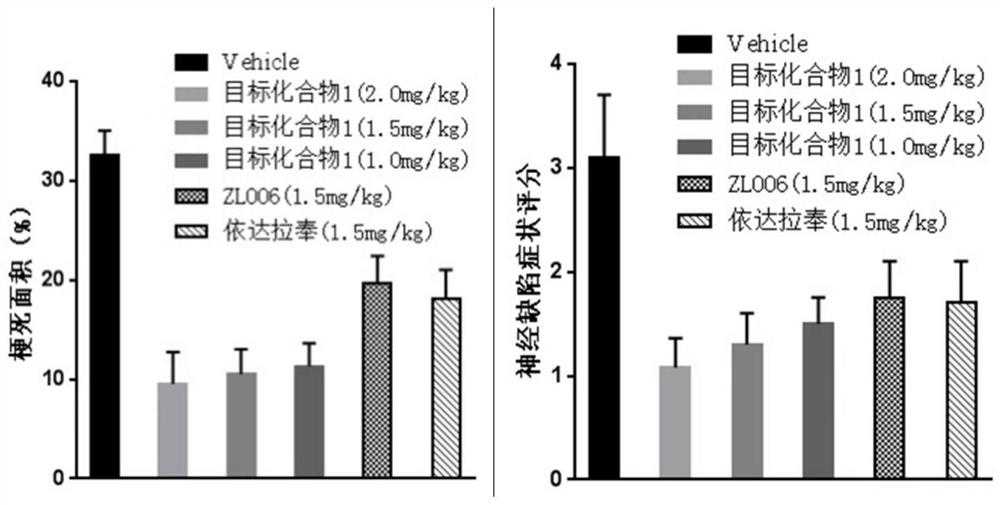
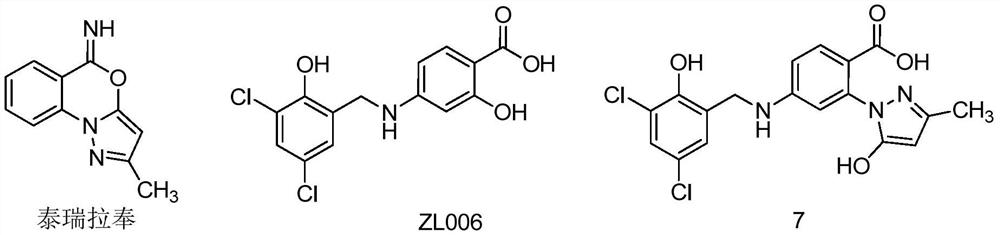
A class of n-benzylaniline derivatives with free radical scavenging effect and their medicinal use
A technology of benzylaniline and free radicals, which is applied in the field of N-benzylaniline derivatives and their pharmaceutical uses, can solve the problems of decreased free radical scavenging ability, decreased benzene ring electron cloud density and the like, and achieves good free radical scavenging activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthesis of embodiment 1 target compound:
[0020] 1.1 Synthesis of target compound 1:
[0021] synthetic route:
[0022]
[0023] Add raw material A (4.3g, 20mmol) and 3,5-dichlorosalicylaldehyde (3.9g, 20mmol) in a 250mL eggplant-shaped bottle, add 100mL methanol, heat and reflux for 12h, filter, and dry the filter cake to obtain Imine light red solid 2.2g, add 50mL methanol, imine (2.2g) in 250mL eggplant-shaped bottle, add sodium borohydride (1.0g) in 5 batches, after TCL detects that the reaction is complete, adjust the pH with concentrated HCl to 7 or so, add water 100mL, a pale yellow solid precipitates out, filter with suction, take the filter cake, and dry to obtain 1.6 g of a pale yellow solid, with a yield of 72.4%. 1 H NMR(400MHz,DMSO-d6)δ9.72(s,1H),7.62(s,1H),7.60(t,1H),7.46(d,1H),7.25(d,1H),6.70(s, 1H), 6.51(d, 1H), 5.55(s, 1H), 4.36(d, 2H), 2.39(s, 3H). 13 C NMR (101MHz, DMSO-D6) δ160.65, 156.98, 155.80, 150.86, 150.24, 141.64, 129.97, 128.11,...
Embodiment 2
[0042] Embodiment 2 target compound anti-DPPH free radical test
[0043] Diphenylpicryl radical (DPPH) is a convenient method for screening antioxidants. When excess free radical scavenger exists, its remaining concentration can reflect the free radical scavenging ability of the target substance, the smaller the remaining concentration, the greater the free radical scavenging ability.
[0044] Accurately weigh 39.4mg of DPPH, place it in a 100mL volumetric flask, and dilute it to 100mL with 95% ethanol to make a DPPH solution (1.0mmol / L). The target compound is prepared as a 1.0mmol / L solution with DMF as a solvent .
[0045] 1.0mL of DPPH solution and 4.0mL of the target compound solution were placed in a 10mL volumetric flask, and the volume was adjusted to 10mL with water, and left for 3 hours, and the concentration of remaining DPPH was measured by high performance liquid phase method (detection wavelength: 517nm).
[0046] The concentration (mmol / L) of the residual DPPH...
Embodiment 3
[0049] Example 3 The protective effect of the target compound on the injury of primary cortical neurons cultured in vitro (glutamic acid model)
[0050] After the primary neurons were cultured for 9 days, they were pre-administered for 30 minutes and incubated for 45 minutes with 50 μmol of glutamate + 10 μmol of glycine. The liquid was changed in full amount (drugs and glutamic acid glycine were all withdrawn), and the LDH leakage rate was measured after 8 hours.
[0051] After the primary cortical neurons were given various treatments, the neuron culture medium in the six-well plate was drawn into a 1.5mL plastic centrifuge tube for later use; the cells were washed with PBS solution 3 times, 1000 μL of PBS was added to each well, and the cells were repeatedly frozen and thawed for 3 centrifuge at 12,000'g at 4°C for 5 minutes, and absorb the supernatant for later use. Aspirate 50 μL of culture solution and 25 μL of supernatant to measure LDH enzyme activity, and use lactate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com