Genetic engineering cell strain of obesity-resistant medicine target point UCP1, establishing and application of high-flux medicine screening model
A technology of target cells and cell lines, applied in genetic engineering, screening of compounds, and cells modified by introducing foreign genetic material, etc., can solve problems such as poor compliance and weight rebound, and reduce the mismatch rate and false positive rate. , the effect of high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1, Cas9 / sgRNA design targeting the UCP1 gene locus of immortalized brown adipocytes
[0066] 1. Sequencing confirmation of UCP1 sequence
[0067] The sequence of UCP1 gene may be different in different cell lines. In order to ensure the efficiency of the designed Cas9 / sgRNA, the UCP1 gene sequence of immortalized brown adipocytes was first amplified by PCR and sequenced to verify that the sgRNA recognition sequence was completely consistent with the UCP1 gene sequence of immortalized brown adipocytes.
[0068] Experimental method: (1) PCR primers are as follows:
[0069]
[0070] (2) The PCR reaction system is as follows:
[0071]
[0072]
[0073] (3) PCR reaction conditions are as follows:
[0074] Enzyme: KOD-FX
[0075] Program: Touchdown PCR
[0076]
[0077] Experimental results: PCR and sequencing of the DNA of the immortalized brown adipocytes showed that the UCP1 gene sequence of the immortalized brown adipocytes was completely consistent...
Embodiment 2
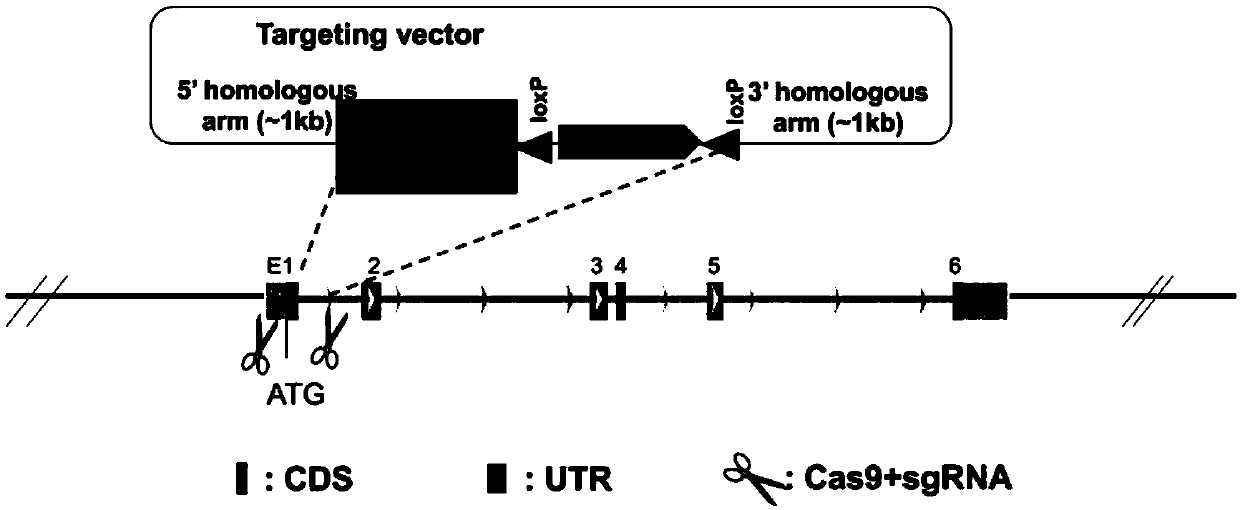
[0085] Embodiment 2, construction and preparation of recombinant exogenous gene carrier
[0086] Synthesize the 5' homologous arm (5'-homologous arm, LR) and the knock-in fragment luciferase-T2A-tdTomato-WPRE-pA (A), clone the 3' homologous arm (3'-homologous arm, RR) fragment , and the LR-A and RR fragments were connected with the carrier LScKO-4G to form a recombinant exogenous gene carrier UCP1-LScKO-4G-Neo-LR-A-RR. After enzyme digestion identification and sequencing, it was confirmed that the recombinant exogenous gene carrier was constructed .
[0087] 1. Exogenous gene cloning
[0088] Experimental method: (1) UCP1-LR-A was prepared by direct synthesis method
[0089]
[0090] (2) UCP1-RR is prepared by PCR method, and the primer sequences are as follows:
[0091]
[0092] (3) The LR-A (EcoRI+XhoI) and RR (NotI+NheI) fragments were connected to the vector LScKO-4G by enzyme digestion and ligation to form a recombinant exogenous gene vector UCP1-LScKO-4G-Neo-LR-...
Embodiment 3
[0105] Embodiment 3, positive clone screening
[0106] 1. Cell transfection
[0107] Experimental method: UCP1-sgRNA1, UCP1-sgRNA6 and recombinant exogenous gene vectors were electroporated to immortalize brown adipocytes. For electroporation methods, refer to the neon transfection instructions.
[0108] 2. Drug screening
[0109] Experimental method: 24 hours after transfection, 600 μg / mL G418 was added for drug screening, and 7 days later, enrichment was carried out to obtain mixed clones.
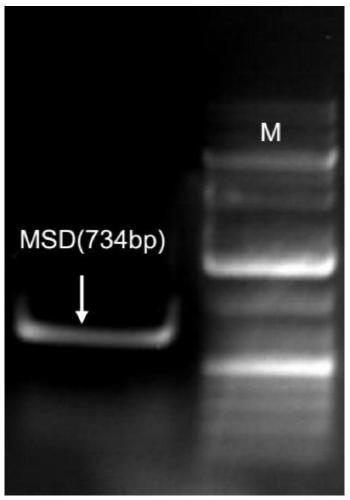
[0110] 3. PCR screening of mixed clones
[0111] Experimental method: Screening of mixed clones by PCR method, the specific process is as follows:
[0112] (1) PCR screening hybrid cloning primer design strategy:
[0113] See details Figure 9 .
[0114] (2) PCR screening primer sequences are as follows:
[0115]
[0116] (3) The PCR reaction system is as follows:
[0117] React components Volume(μL) final concentration ddH2O 19 — 2×KOD FX buffer 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com