A kind of method for preparing m-phenylenediamine
A technology of m-phenylenediamine and isophthalamide, which is applied in the preparation of carboxylic esters, carboxylic acid amides, and oxidized carboxylic acids. Scale experiments and other issues to achieve the effect of avoiding dangerous processes and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
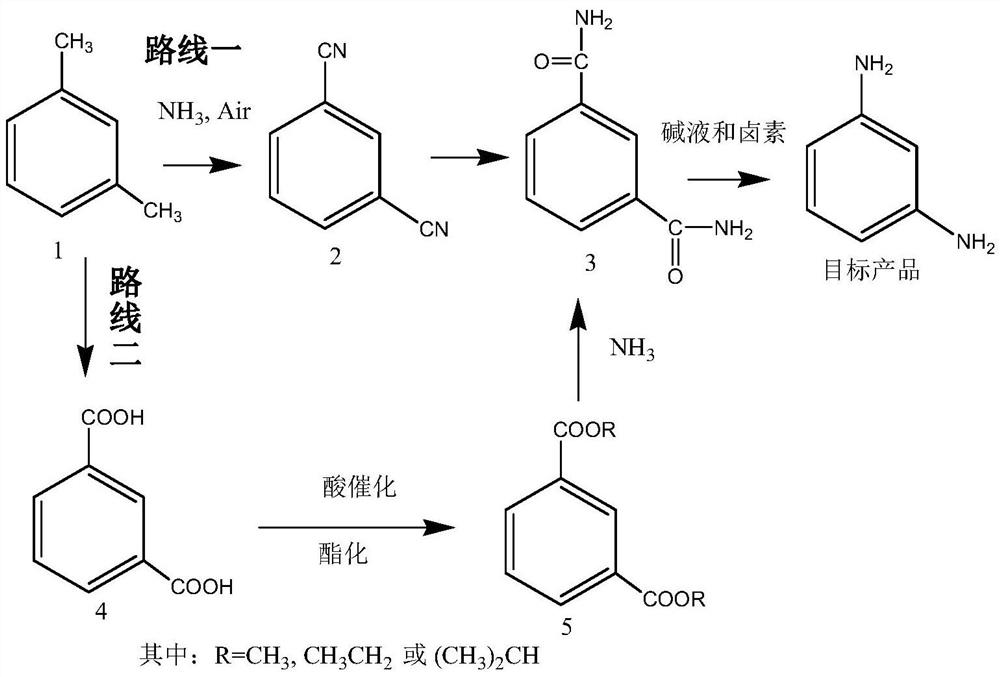
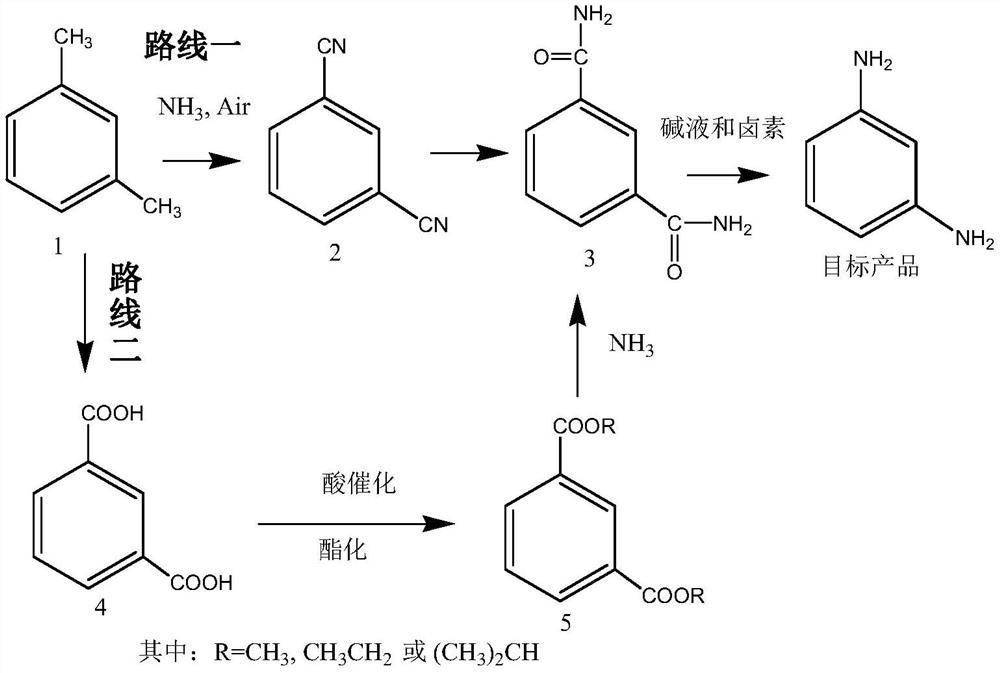
[0081] Embodiment 1 (route one)
[0082] The first step: the preparation of isophthalonitrile (compound 2)
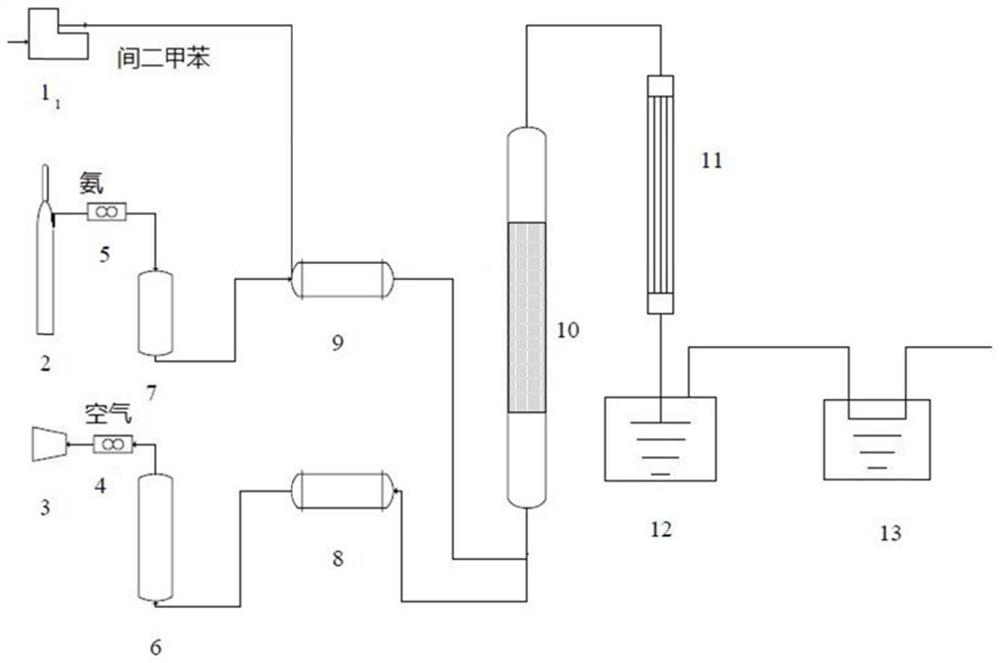
[0083] use figure 1 The device prepares isophthalonitrile. In the figure, 1 is a micropump, 2 is an ammonia gas source, 3 is an air compressor, 4 is an air flow meter, 5 is an ammonia gas flow meter, 6 is an air buffer tank, 7 is an ammonia gas buffer tank, and 8 is an air preheating tank. Heater, 9 is an ammonia gas preheater, 10 is a fixed bed reactor, 11 is a condenser, 12 is a receiving tank, and 13 is an acid liquid tank. Ammonia gas source 2, ammonia gas flowmeter 5, ammonia gas buffer tank 7 and ammonia gas preheater 9 are connected in sequence, and air compressor 3, air flow meter 4, air buffer tank 6 and air preheater 8 are connected in sequence, and the air The outlet of preheater 8 and air preheater 8 is connected with fixed bed reactor 10, and fixed bed reactor 10 is connected with condenser 11, receiving tank 12 and acid liquid tank 13 successively, and ...
Embodiment 2
[0085] Route one: the second step: the preparation of isophthalamide (compound 3)
[0086] Dissolve 128 g of isophthalonitrile in 600 ml of methanol solution, add 20 g of sodium carbonate aqueous solution, heat up and reflux for 5 to 6 hours to reach the end point, evaporate methanol to dryness, and recrystallize to obtain 156 g of isophthalamide (compound 3). Yield 95.1%.
Embodiment 3
[0087] The preparation of embodiment 3 isophthalamide (compound 3)
[0088] Isophthalonitrile 128g is dissolved in 500ml ethanol solution, adds 5g sodium hydroxide, adds dropwise 50ml ammoniacal liquor, heats up and refluxes and reaches the end point after 5~6 hours, evaporates solvent, and recrystallization obtains 157.6g isophthalamide (compound 3). Yield 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com